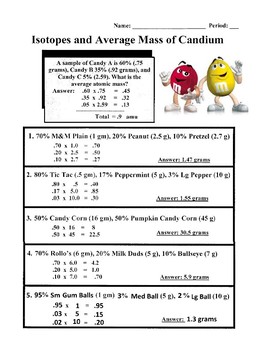
45 isotopes and average atomic mass worksheet answers
› cms › libIsotope Worksheet Answer Key - ISD 622 mass # a35 33 # of protons 19 # of neutrons 22 Isoto e name uranium-235 uranium-23 8 boron- 10 boron-11 atomic # Phos hocus-33 15 Write the hyphen notation and the nuclide (nuclear) symbol for an isotope that has 17 protons, 17 electrons, and 20 neutrons. 37 Isotopes are atoms of the same element with a different number of › createJoin LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols;
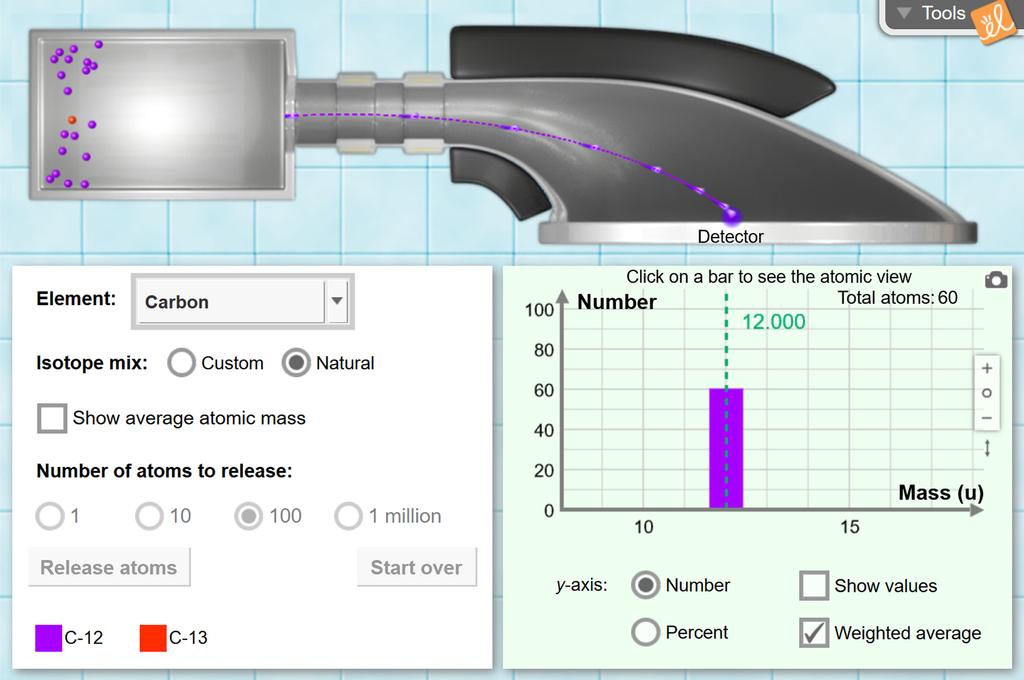
phet.colorado.edu › sims › htmlIsotopes and Atomic Mass - PhET Isotopes and Atomic Mass - PhET
Isotopes and average atomic mass worksheet answers
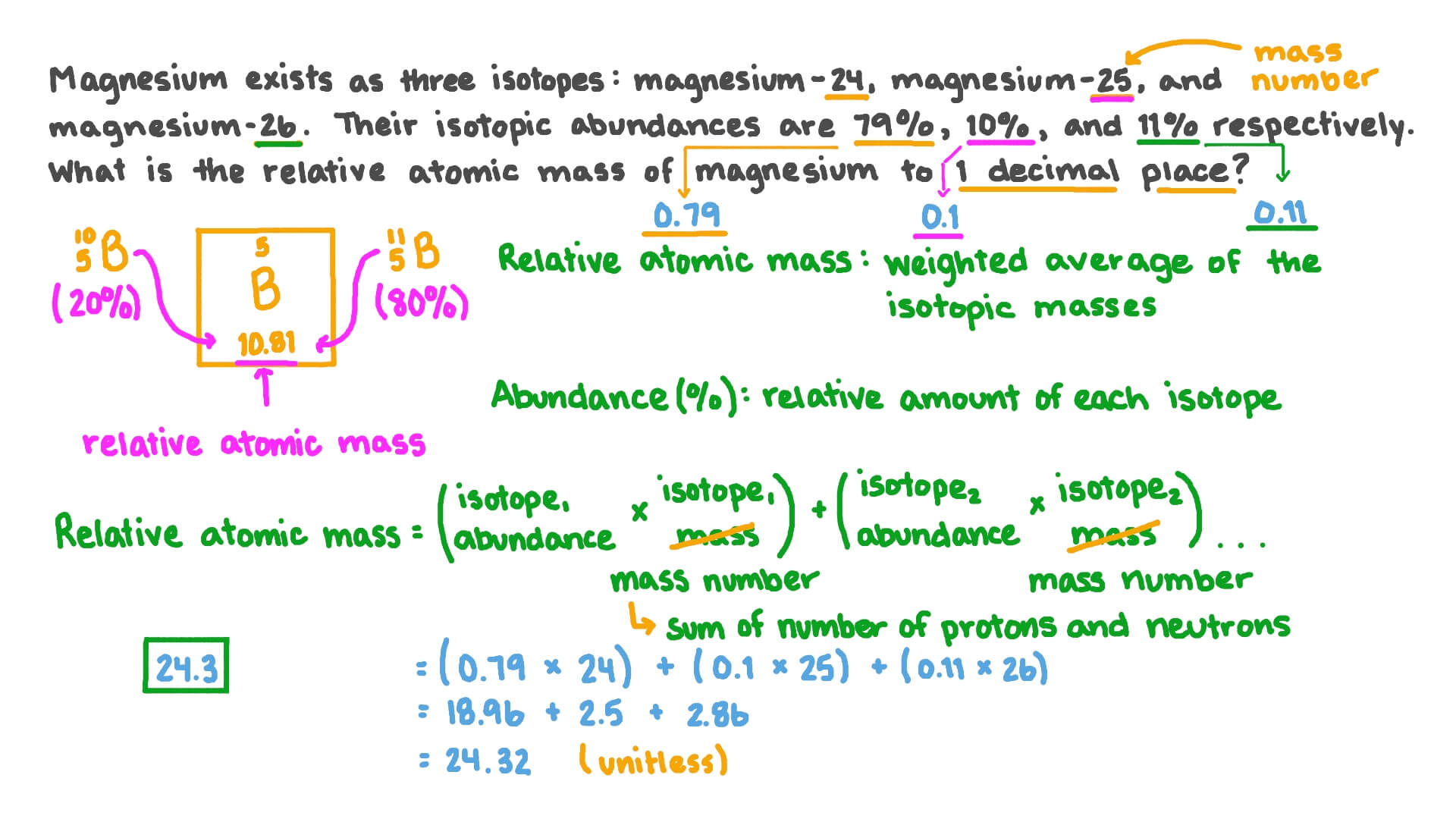
study.com › academy › practiceQuiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element? coursehelponline.comCourse Help Online - Have your academic paper written by a ... 100% money-back guarantee. With our money back guarantee, our customers have the right to request and get a refund at any stage of their order in case something goes wrong. › relative-atomic-massIsotopes & Relative Atomic Mass (solutions, examples, videos) An atom of carbon-12 is taken to have a mass of 12 atomic mass unit (amu). Since one carbon-12 atom has 6 proton and 6 neutron, mass of one proton (neutron) = mass of one carbon-12 atom = 1 amu (atomic mass unit) Atomic Mass Units. Average Atomic Mass and definition of atomic mass unit. Show Video Lesson
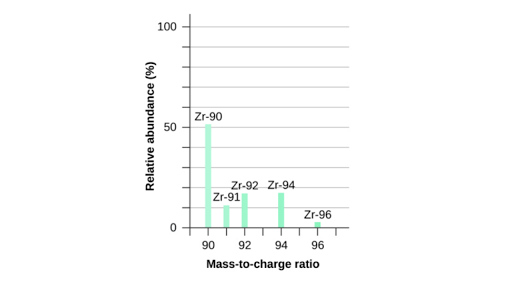
Isotopes and average atomic mass worksheet answers. clc.chem.wisc.edu › clc-chemistry-resourcesCLC Chemistry Resources – Chemistry Learning Center – UW–Madison Atomic Structure Atomic Structure, Isotopes, Average Atomic Mass, Mass Spectroscopy, Ionic versus Molecular compounds, Naming/Writing Formulas, Polyatomic Ions, Coulomb’s Law: Flashcards #3 Polyatomic Ions Formulas/Names Confirm which polyatomic ions you are expected to know in your course; print 2-sided from Adobe Reader for proper alignment › relative-atomic-massIsotopes & Relative Atomic Mass (solutions, examples, videos) An atom of carbon-12 is taken to have a mass of 12 atomic mass unit (amu). Since one carbon-12 atom has 6 proton and 6 neutron, mass of one proton (neutron) = mass of one carbon-12 atom = 1 amu (atomic mass unit) Atomic Mass Units. Average Atomic Mass and definition of atomic mass unit. Show Video Lesson coursehelponline.comCourse Help Online - Have your academic paper written by a ... 100% money-back guarantee. With our money back guarantee, our customers have the right to request and get a refund at any stage of their order in case something goes wrong. study.com › academy › practiceQuiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element?


























0 Response to "45 isotopes and average atomic mass worksheet answers"
Post a Comment