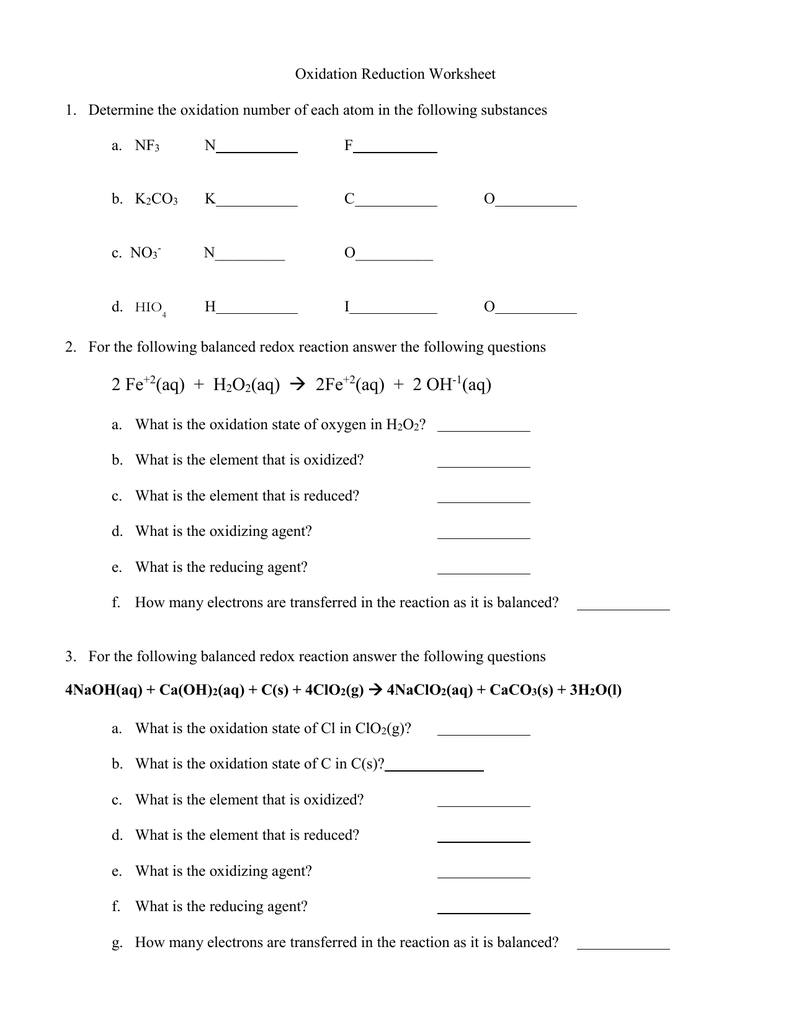
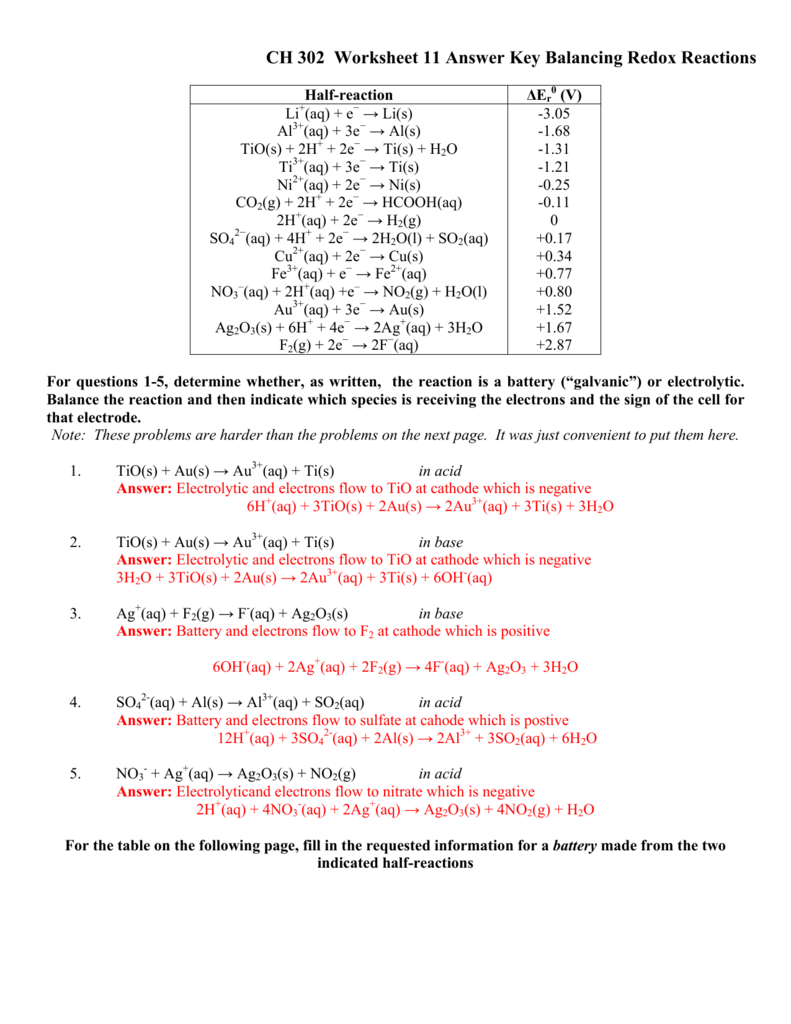
41 oxidation reduction reactions worksheet answers
Chemistry 12 Worksheet 4-6 ANHYDRIDES, ACID RAIN AND TITRATIONS KEYp1 p2 p3 p4 p5 p6. Worksheet 4-7 ACID-BASE INDICATORS KEY p 1 p 2 p3. Experiment 20-D Hydrolysis. Unit 5. Redox. DETAILED NOTES ON OXIDATION - REDUCTION INTRODUCTION AND TERMS, OXIDATION NUMBERS, THE REDOX TABLE, PREDICTING REDOX REACTIONS Unit 5 Notes P1-16. VIDEO ON DETERMINING … Chemistry - Wikipedia Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances.. In the scope of its subject, chemistry occupies an …
Electric Circuits Review - Answers #2 - Physics Classroom Batteries perform their energy-supplying tasks by using the energy from an exothermic oxidation-reduction reaction to do work upon charge within the electric circuit. When a battery no longer works, its reactants are consumed to the point that the electric potential which the reactants are capable of producing is small compared to the overall ...

Oxidation reduction reactions worksheet answers
Electrolysis of molten lead(II) bromide | Experiment | RSC ... Introduction to oxidation and reduction: simple examples only, e.g. Na with Cl₂, Mg with O₂, Zn with Cu²⁺. Oxidation and reduction in terms of loss and gain of electrons. The electrochemical series as a series of metals arranged in order of their ability to be oxidised (reactions, other than displacement reactions, not required). Momentum and Collisions Review - with Answers #2 Answer: D. In any collision, there are always four quantities which are the same for both objects involved in the collision. Each object experiences the same force (Newton's third law) for the same amount of time, leading to the same impulse, and subsequently the same momentum change. Worksheet # 5 Balancing Redox Reactions in Acid and Basic ... State of the change that represents oxidation, reduction or neither. Use oxidation #s. Remember that if the oxidation # increases it means oxidation and when it decreases it mean reduction! 18. MnO 2 → Mn 2O 3 19. NH 3 → NO 2 20. HClO 4 → HCl + H 2O 21. O 2 → O2-22. P 2O 5 → P 4H 10 Determine the oxidation number 23. H 2SO 4 22. HSO 4 ...
Oxidation reduction reactions worksheet answers. Reactions of metals with acids producing salts - RSC Education 4.3 Explain the reactivity series of metals (potassium, sodium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen), copper, silver, gold) in terms of the reactivity of the metals with water and dilute acids and that these reactions show the… OCR Chemistry A: Gateway. C3 Chemical reactions. C3.3 Types of chemical reactions Redox Reactions Practice Worksheet with Answers May 08, 2013 · B. reduction, only C. both oxidation and reduction D. neither oxidation nor reduction 23. In the reaction AgNO3(aq)+NaCl(aq) !NaNO3(aq)+AgCl(s), the reactants A. gain electrons, only B. lose electrons, only C. both gain and lose electrons D. neither gain nor lose electrons 24. In the reaction Mg+Cl2!MgCl2, the correct half-reaction for the ... Worksheet # 5 Balancing Redox Reactions in Acid and Basic ... State of the change that represents oxidation, reduction or neither. Use oxidation #s. Remember that if the oxidation # increases it means oxidation and when it decreases it mean reduction! 18. MnO 2 → Mn 2O 3 19. NH 3 → NO 2 20. HClO 4 → HCl + H 2O 21. O 2 → O2-22. P 2O 5 → P 4H 10 Determine the oxidation number 23. H 2SO 4 22. HSO 4 ... Momentum and Collisions Review - with Answers #2 Answer: D. In any collision, there are always four quantities which are the same for both objects involved in the collision. Each object experiences the same force (Newton's third law) for the same amount of time, leading to the same impulse, and subsequently the same momentum change.
Electrolysis of molten lead(II) bromide | Experiment | RSC ... Introduction to oxidation and reduction: simple examples only, e.g. Na with Cl₂, Mg with O₂, Zn with Cu²⁺. Oxidation and reduction in terms of loss and gain of electrons. The electrochemical series as a series of metals arranged in order of their ability to be oxidised (reactions, other than displacement reactions, not required).































0 Response to "41 oxidation reduction reactions worksheet answers"
Post a Comment