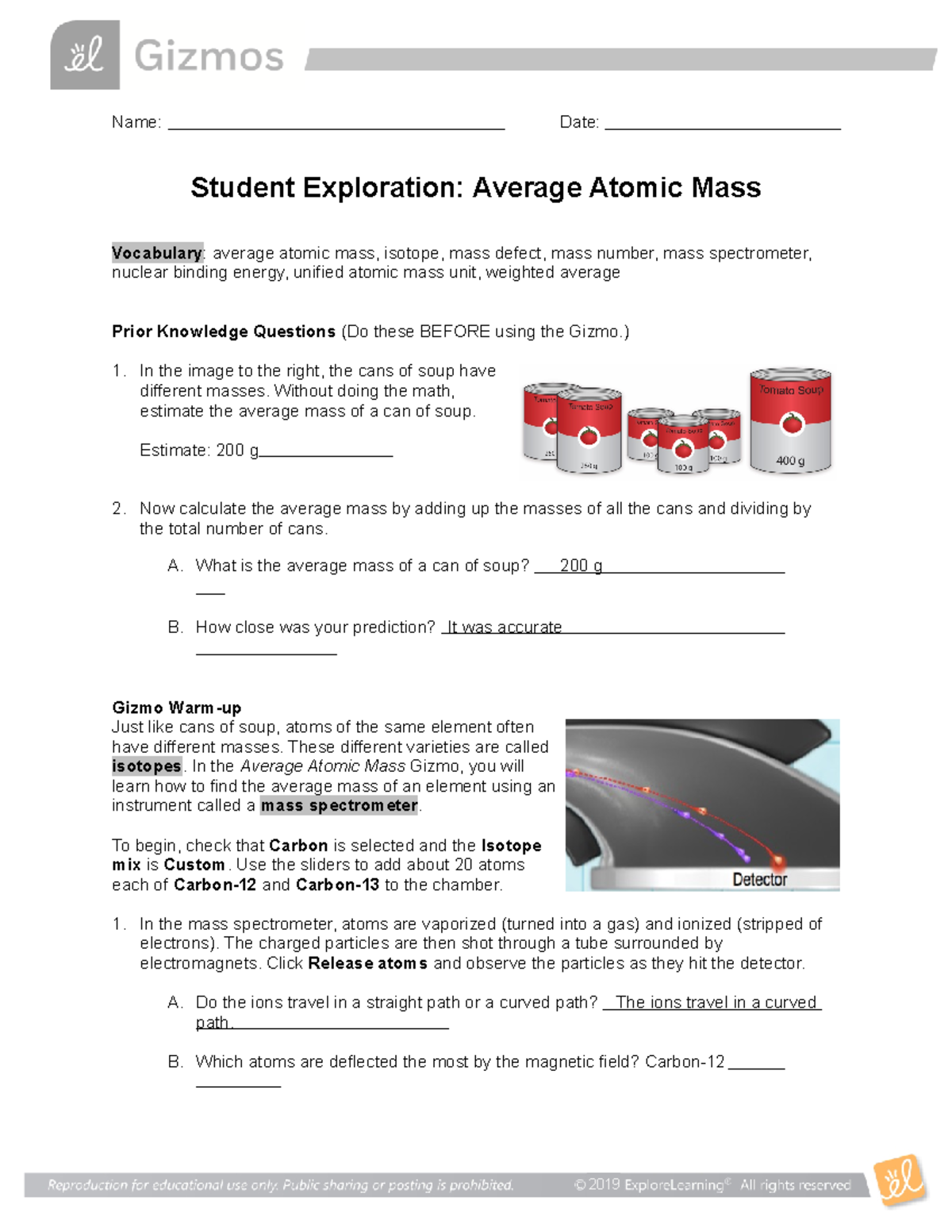
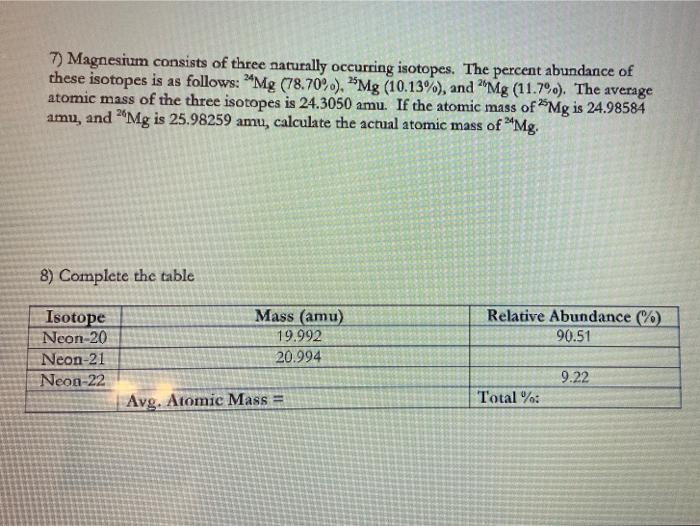
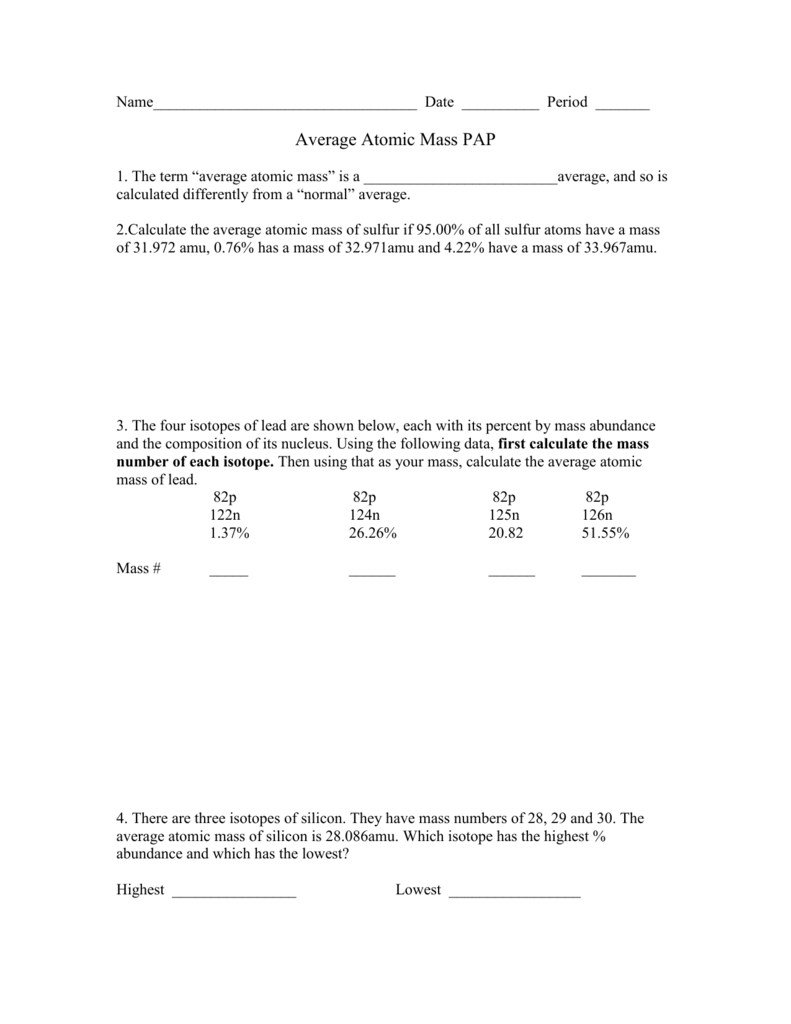
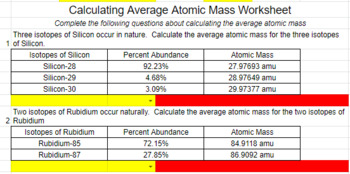
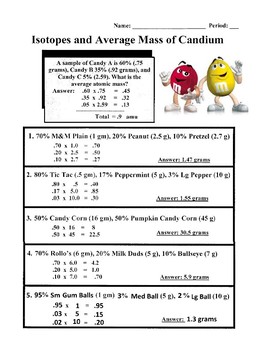
38 isotopes and average atomic mass worksheet
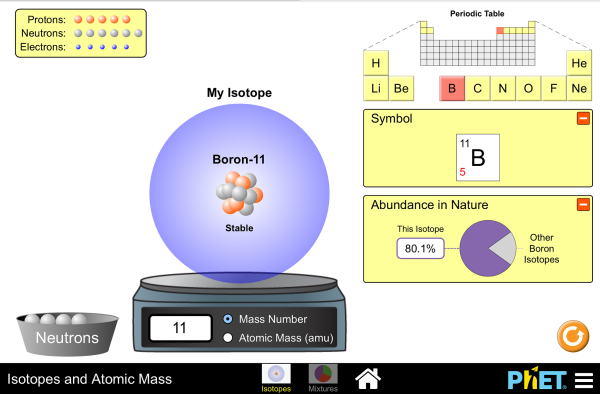
Isotopes & Relative Atomic Mass (solutions, examples, videos) An atom of carbon-12 is taken to have a mass of 12 atomic mass unit (amu). Since one carbon-12 atom has 6 proton and 6 neutron, mass of one proton (neutron) = mass of one carbon-12 atom = 1 amu (atomic mass unit) Atomic Mass Units. Average Atomic Mass and definition of atomic mass unit. Show Video Lesson phet.colorado.edu › sims › htmlIsotopes and Atomic Mass - PhET Isotopes and Atomic Mass - PhET
Build an Atom - Atoms | Atomic Structure | Isotope Symbols Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!

Isotopes and average atomic mass worksheet
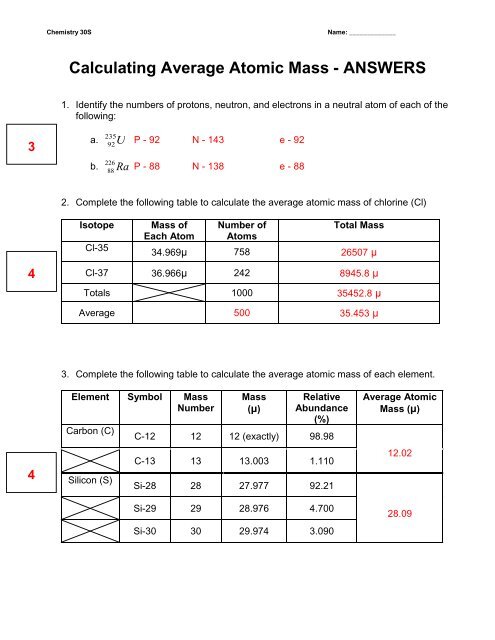
Molecular Mass Calculations - ThoughtCo 11.03.2019 · Note About Molecular Mass and Isotopes . The molecular mass calculations made using the atomic masses on the periodic table apply for general calculations, but aren't accurate when known isotopes of atoms are present in a compound. This is because the periodic table lists values that are a weighted average of the mass of all natural isotopes of each … study.com › skill › learnHow to Calculate Average Atomic Mass | Chemistry | Study.com How to Calculate Average Atomic Mass: Example 1. Calculate the average atomic mass of boron given that 19.8% of its naturally occurring atoms have a mass of 10.013 amu and 80.2% have a mass of 11. ... Basic Atomic Structure Worksheet Key - Neshaminy School District in a neutral atom of that element. The atomic number gives the "identity" of an element as well as its location on the periodic table. No two different elements will have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope.
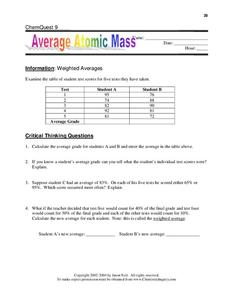
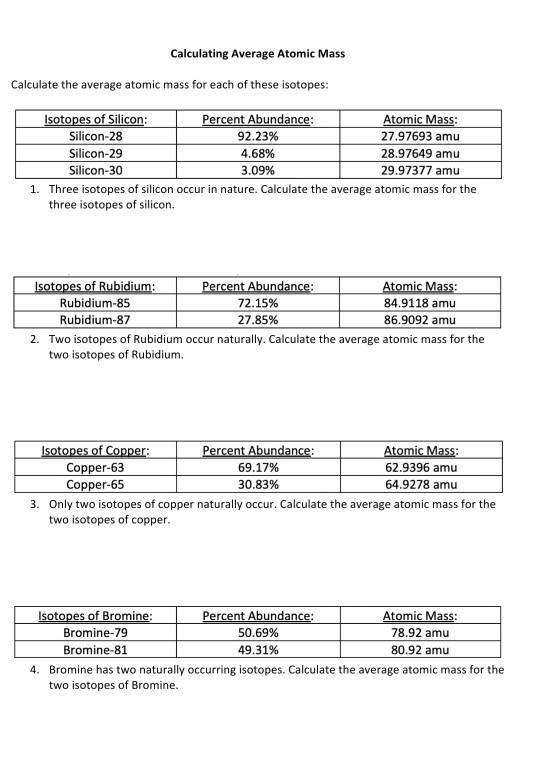
Isotopes and average atomic mass worksheet. › en-us › documentCalculating Average Atomic Mass Worksheet - Name - StuDocu Calculating Average Atomic Mass Worksheet. Show ALL calculation setups. The term “average atomic mass” is a _____average, and so is calculated differently, from a “normal” average. Explain how this type of average is calculated.-----The element Copper has naturally occurring isotopes with mass numbers of 63 and 65. achieverpapers.comAchiever Papers - We help students improve their academic ... 100% money-back guarantee. With our money back guarantee, our customers have the right to request and get a refund at any stage of their order in case something goes wrong. › relative-atomic-massIsotopes & Relative Atomic Mass (solutions, examples, videos) An atom of carbon-12 is taken to have a mass of 12 atomic mass unit (amu). Since one carbon-12 atom has 6 proton and 6 neutron, mass of one proton (neutron) = mass of one carbon-12 atom = 1 amu (atomic mass unit) Atomic Mass Units. Average Atomic Mass and definition of atomic mass unit. Show Video Lesson study.com › skill › learnIdentifying Elements and Masses Using a Mass Spectrum of an ... Aluminum has an average atomic mass of 26.98 amu and phosphorus has an average atomic mass of 30.97 amu. Neither of the other masses would make sense with a major isotope of 28 amu. This element ...
› createJoin LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; Chem4Kids.com: Elements & Periodic Table: Periodic Table Atomic hydrogen wants to combine with other elements to fill its outer shell. Your chemistry work will most likley use molecular hydrogen (H 2) or hydrogen ions (H +, protons). Helium (He) is different from all of the other elements. It is very stable with only two electrons in its outer orbital (valence shell). Even though it only has two electrons, it is still grouped with the noble gases ... Basic Atomic Structure Worksheet Key - Neshaminy School District in a neutral atom of that element. The atomic number gives the "identity" of an element as well as its location on the periodic table. No two different elements will have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. study.com › skill › learnHow to Calculate Average Atomic Mass | Chemistry | Study.com How to Calculate Average Atomic Mass: Example 1. Calculate the average atomic mass of boron given that 19.8% of its naturally occurring atoms have a mass of 10.013 amu and 80.2% have a mass of 11. ...
Molecular Mass Calculations - ThoughtCo 11.03.2019 · Note About Molecular Mass and Isotopes . The molecular mass calculations made using the atomic masses on the periodic table apply for general calculations, but aren't accurate when known isotopes of atoms are present in a compound. This is because the periodic table lists values that are a weighted average of the mass of all natural isotopes of each …




















0 Response to "38 isotopes and average atomic mass worksheet"
Post a Comment