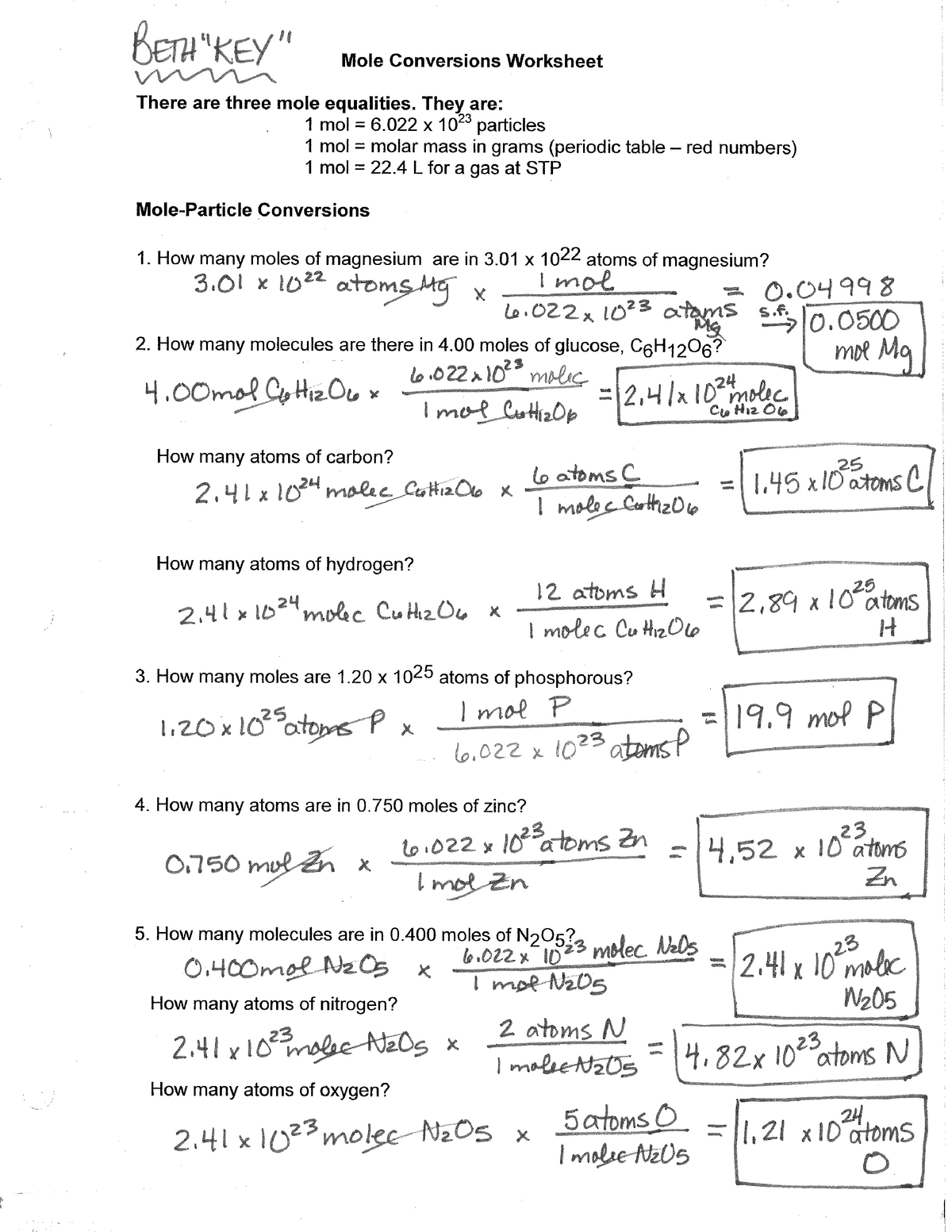
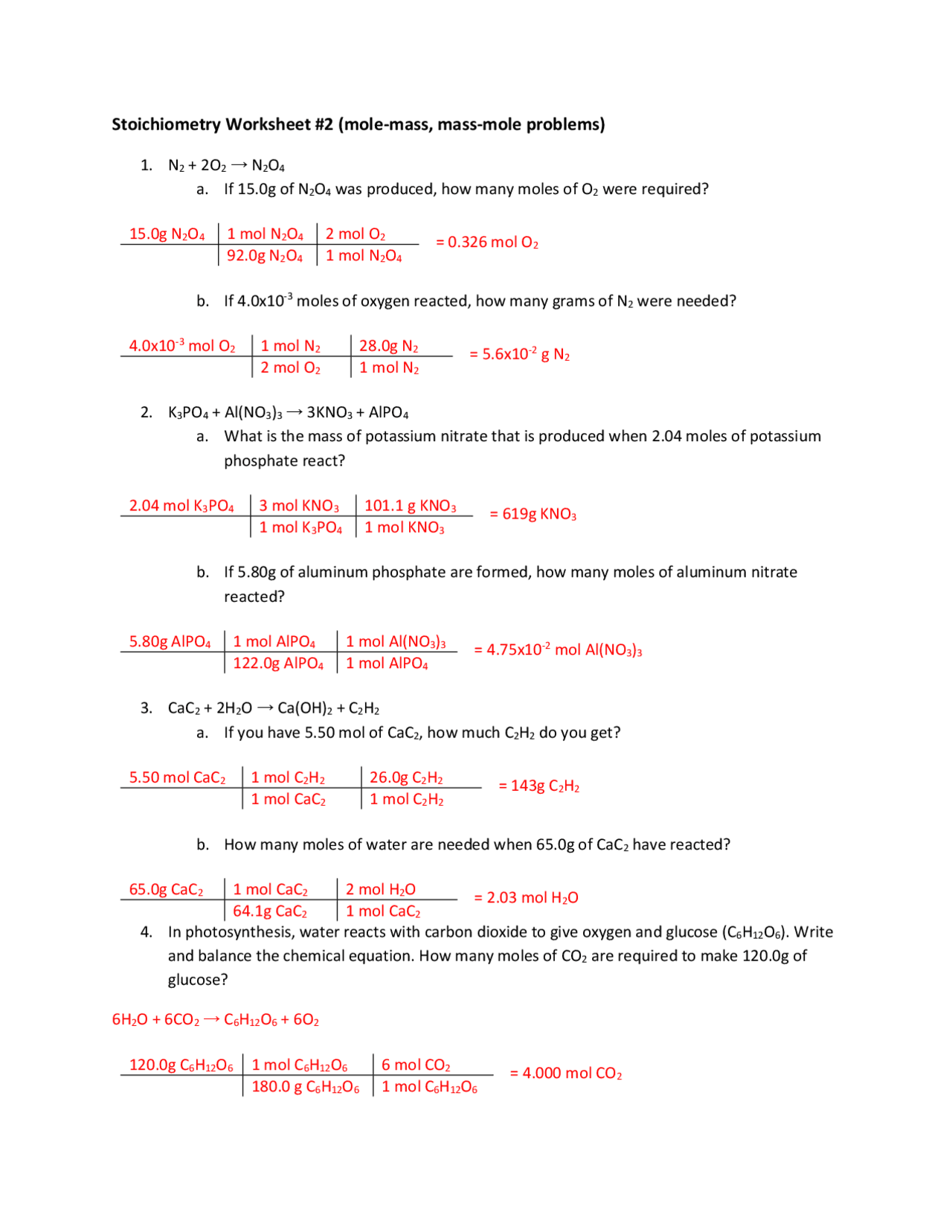
44 mole problems chemistry worksheet with answers
Chemistry Worksheets and Handouts (PDF for Printing) Mar 08, 2021 · Print free chemistry worksheets and handouts to enhance student learning. This is a collection of free chemistry worksheets and handouts to print. Most of the printables are PDF files, although some are available as JPG or PNG files. Chemistry Worksheets. Label Parts of the Atom [Google Apps worksheet][worksheet PDF][worksheet PNG][answers PNG] How to Calculate Mass Percent Composition - ThoughtCo Nov 24, 2019 · For a solution, mass percent equals the mass of an element in one mole of the compound divided by the molar mass of the compound, multiplied by 100%. Mass Percent Composition Problem Bicarbonate of soda ( sodium hydrogen carbonate ) is used in many commercial preparations.
Worked Chemistry Problem Examples - ThoughtCo Nov 22, 2019 · Alphabetical Index of Chemistry Problem Types . Included in this list are printable pdf chemistry worksheets so you can practice problems and then check your answers. You may also browse chemistry problems according to the type of problem.

Mole problems chemistry worksheet with answers
Freezing and Boiling Points - CliffsNotes 0.3 kg × 0.676 mole/kg = 0.203 mole. and the molecular weight is calculated as The boiling point of a solution was used to determine that santonic acid has a molecular mass of approximately 246. You can also find this value by using the freezing point of the solution. Mole Concept- Formula, Explanations, Examples, Related ... Mole Concept- A mole is defined as the amount of a substance that contains exactly the Avogadro number of ‘elementary entities’ of the given substance. The Avogadro number is represented by NA. The Mole Concept is a Convenient Method of Expressing the Amount of a Substance. To Learn more about the Mole Concept with Formulae and Examples with Videos and FAQs, The number of electrons in a ... Calculating pH and pOH worksheet - Everett Community College Since there is both acid and base we will assume a 1 mole acid:1 mole base ratio of neutralization. There is more base than acid so the leftover base is what will affect the pH of the solution. 3.60 x 10-3 moles - 5.95 x 10-4 moles = 3.01 x 10-3 moles NaOH 3.01 x 10-3 moles NaOH = 3.01 x 10-3 M NaOH 1.00 L soln pOH = -log[OH-] = -log(3.01 x 10 ...
Mole problems chemistry worksheet with answers. Molality, Molarity, Mole fraction: Numerical problems Jan 30, 2020 · Mole fraction of solute (sugarl) = x B = n B /(n A + n B) = 0.1/10.1 = 0.0099. Mole fraction of sugar = 0.0099. Ans: Molality of solution = 0.5556 mol kg-1 and mole fraction of sugar = 0.0099. Example – 04: 10.0 g KCl is dissolved in 1000 g of water. If the density of the solution is 0.997 g cm-3, calculate a) molarity and b) molality of the ... Calculating pH and pOH worksheet - Everett Community College Since there is both acid and base we will assume a 1 mole acid:1 mole base ratio of neutralization. There is more base than acid so the leftover base is what will affect the pH of the solution. 3.60 x 10-3 moles - 5.95 x 10-4 moles = 3.01 x 10-3 moles NaOH 3.01 x 10-3 moles NaOH = 3.01 x 10-3 M NaOH 1.00 L soln pOH = -log[OH-] = -log(3.01 x 10 ... Mole Concept- Formula, Explanations, Examples, Related ... Mole Concept- A mole is defined as the amount of a substance that contains exactly the Avogadro number of ‘elementary entities’ of the given substance. The Avogadro number is represented by NA. The Mole Concept is a Convenient Method of Expressing the Amount of a Substance. To Learn more about the Mole Concept with Formulae and Examples with Videos and FAQs, The number of electrons in a ... Freezing and Boiling Points - CliffsNotes 0.3 kg × 0.676 mole/kg = 0.203 mole. and the molecular weight is calculated as The boiling point of a solution was used to determine that santonic acid has a molecular mass of approximately 246. You can also find this value by using the freezing point of the solution.































0 Response to "44 mole problems chemistry worksheet with answers"
Post a Comment