42 isotopes and atomic mass worksheet answers
Worksheet #4 – Isotopes & Average Atomic Mass Worksheet #1 – Calculations with Subatomic Particles ... Answer the following questions regarding average atomic mass and isotopes. Isotopes and Atomic Mass - What particles determine the ... Isotopes and Atomic Mass gizmo lab sch3u1 privitera name: exploring isotopes and atomic mass phet simulation part making isotopes: open the isotopes screen,
Isotopes Ions And Atoms Worksheet 1 And 2 Answer Key ISOTOPES, IONS, AND ATOMS WORKSHEET. Atomic # = # of protons. Mass # = Atomic # + neutrons. ... charge. Symbol. 1) 17. 19. 0. 2). Ions and Atoms WS-1.pdf Subatomic Particles For Atoms, Ions And Isotopes Answer Sheet Use your periodic table to complete this worksheet. Atom/Ion/Isotope.
Isotopes and atomic mass worksheet answers
Phet Isotopes Atomic And Worksheet Mass Answer [DS0CUV] Average Atomic Mass Worksheet 40 The Of an element is the total number Of protons and neutrons in the Of the atom isotopes and average atomic mass answer key cyteen de 98 #1-9 due at start of class Friday • explain the similarities and differences between isotopes of an element • explain the similarities and differences between isotopes of an element. Atoms And Isotopes Worksheet Answer Key They've the identical variety of protons and September 02, 2021 in isotopes, wallpaper, worksheet. Supply: nidecmege.blogspot.com. June 08, 2021 atoms and isotopes worksheet reply key 5 protons. September 02, 2021 in isotopes, wallpaper, worksheet. Supply: . Phet isotopes and atomic mass worksheet reply key from. 9PS - Atomic Number, Mass Number, Isotopes, and Stuff Atomic Number, Mass Number, Isotopes, and Stuff. 1 Complete the following questions. Assume all atoms are neutral. 56 Fe. He. 26. 2 element: element: atomic ...4 pages
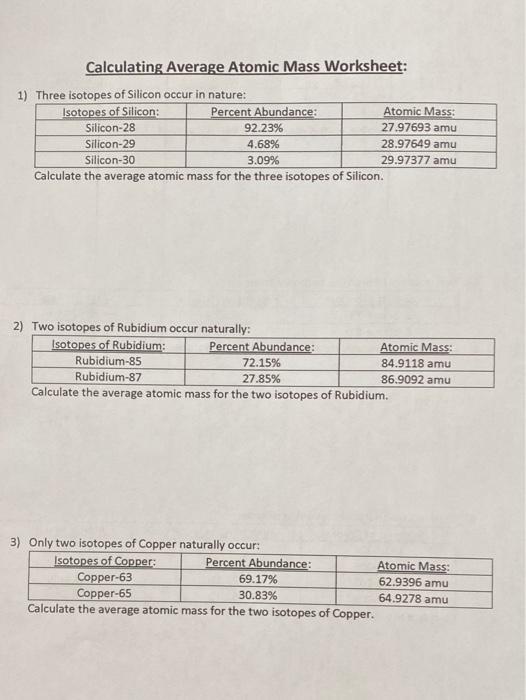
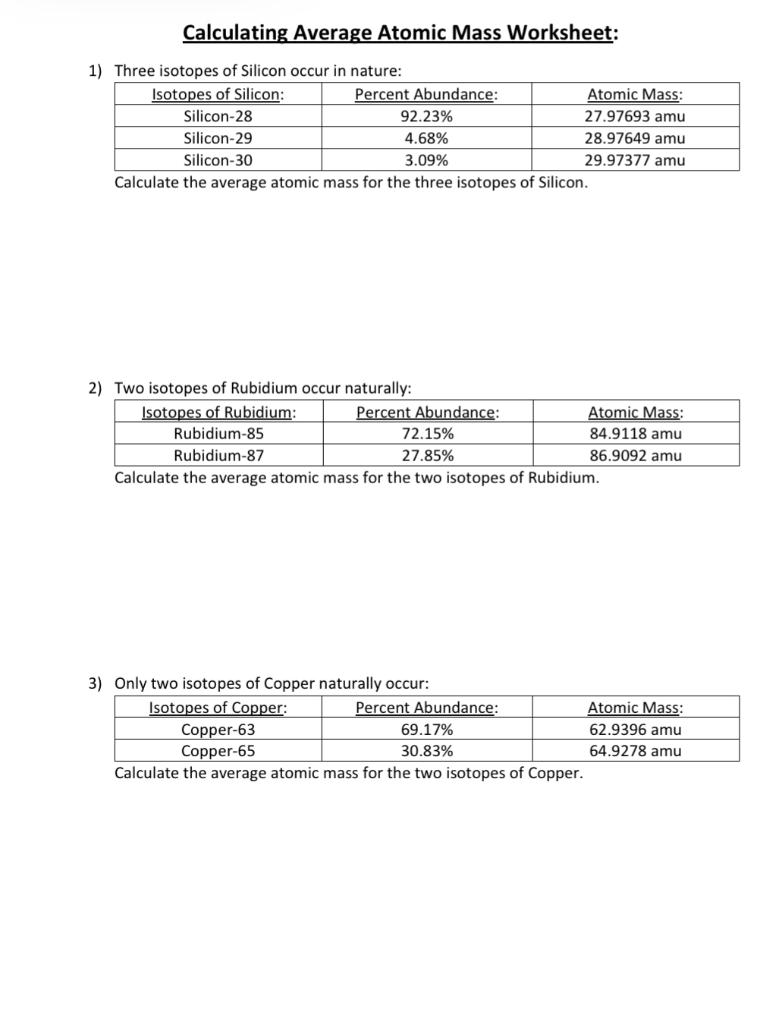
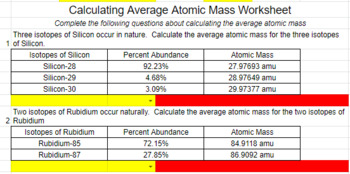
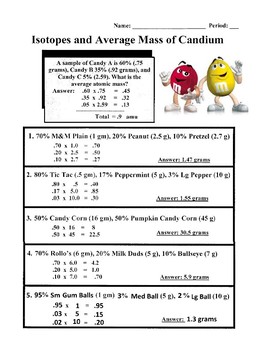
Isotopes and atomic mass worksheet answers. Calculating Average Atomic Mass Worksheet Calculating Average Atomic Mass Worksheet. Teacher. 1. The element copper has naturally occurring isotopes with mass numbers of 63 and 65.2 pages Average Atomic Mass Practice Problems Quiz - Quizizz 6 Questions Show answers Question 1 900 seconds Q. 24.1% of all the isotopes of a an element have a mass of 75.23 amu, 48.7% have a mass of 74.61 amu, and 27.2% have a mass of 75.20 amu. What is the average mass of this element? answer choices 74.92 amu 24.97 amu 75.01 amu 74.51 amu Question 2 120 seconds Q. Calcium has three different isotopes. Molecular Mass Calculations - ThoughtCo 11.3.2019 · Note About Molecular Mass and Isotopes . The molecular mass calculations made using the atomic masses on the periodic table apply for general calculations, but aren't accurate when known isotopes of atoms are present in a compound. This is because the periodic table lists values that are a weighted average of the mass of all natural isotopes of each element. radioactive isotope | Description, Uses, & Examples | Britannica 20.9.2022 · radioactive isotope, also called radioisotope, radionuclide, or radioactive nuclide, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess energy by spontaneously emitting radiation in the form of alpha, beta, and gamma rays. A brief treatment of radioactive isotopes follows. For full treatment, see isotope: …
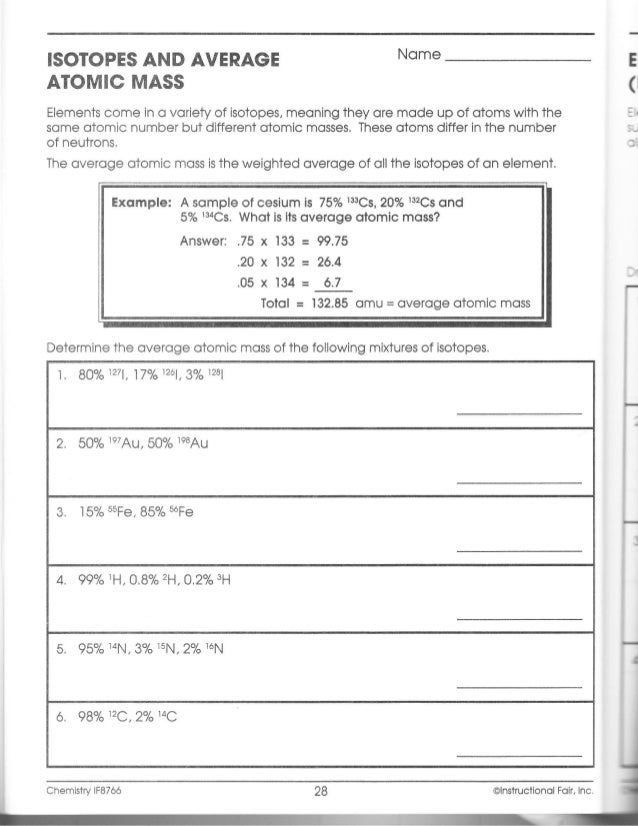
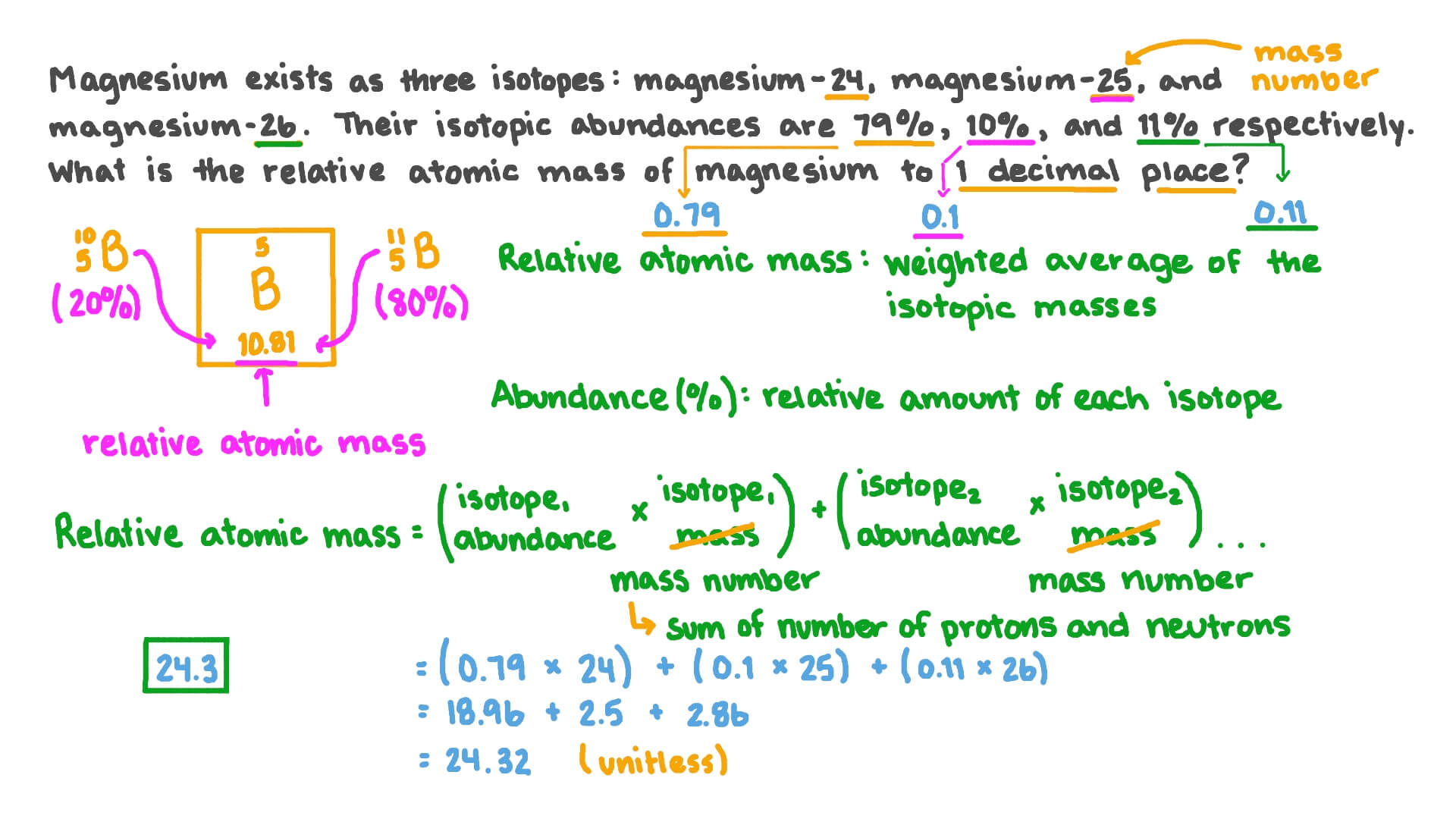
Atoms And Elements Isotopes Worksheet Answers Basic Atomic Structure Worksheet Key 1 gives the "identity" of an element as well as its location on the periodic table. No two different elements will have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. Isotopes & Relative Atomic Mass (solutions, examples, videos) Relative atomic mass. The relative atomic mass ( Ar) of an element is the average mass of the naturally occurring atoms of the element. This quantity takes into account the percentage abundance of all the isotopes of an element which exist. Given that the percentage abundance of is 75% and that of is 25%, calculate the A r of chlorine. PDF KMBT 654-20131024112244 - Berger's Chemistry Class Answer: .75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = Total = 132.85 amu = average atomic mass Determine the average atomic mass of the following mixtures of isotopes. l. IZC 2. 500/0 3. 150/0 4. Il-I, 0.8%4-1, 0.2% 0.01b 5. OMS 13, B 0t46 6. 980/0 Chemistry IF87ó6 CD 04 ©lnstructional Fair, Inc. t). 002-8 3 = 28 Answer Isotopes Worksheet Phet Atomic Mass And [UZ9MDH] The Phet Isotopes and Atomic Mass Worksheet Answer Key are one of the most difficult questions in the latest online study from Robert Heinlein Molecular mass (molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units Weights of atoms and isotopes are from NIST article .
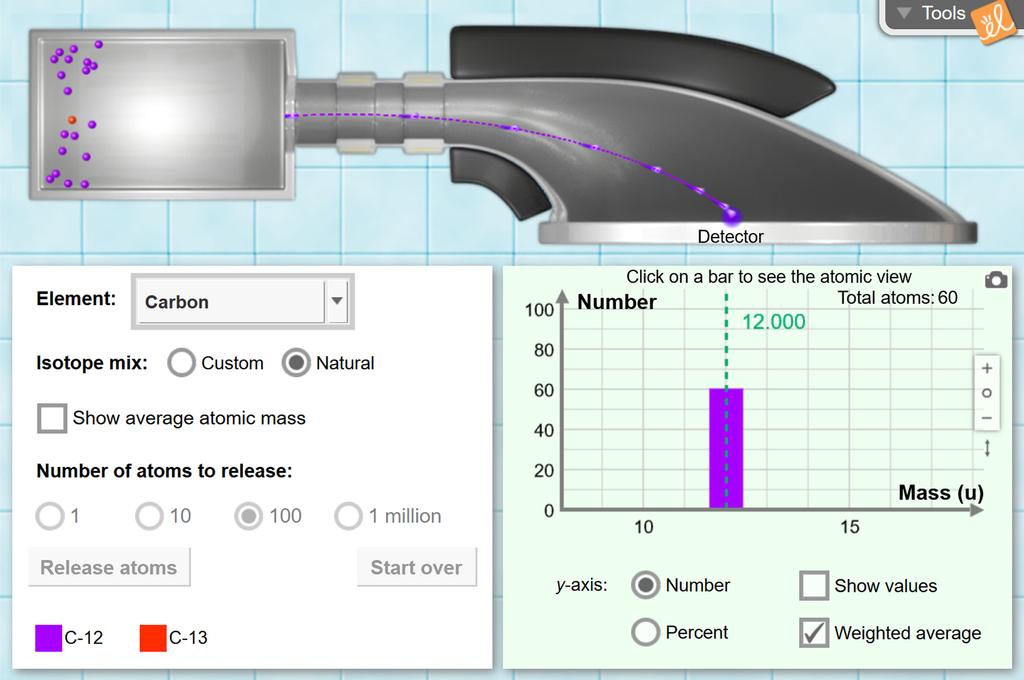
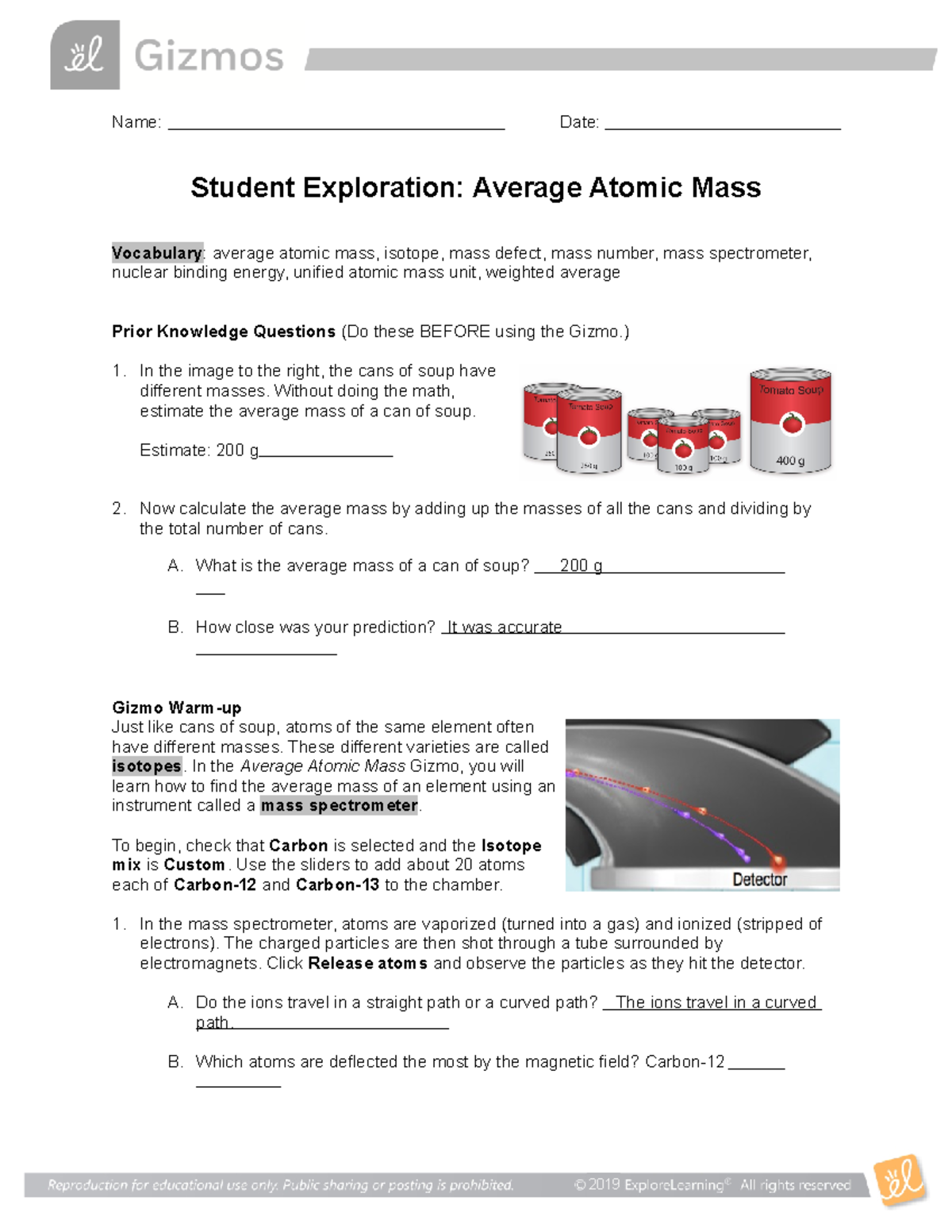
Isotopes and Atomic Mass - Isotopes | Atomic Mass - PhET Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. Are all atoms of an element the same? How can you tell one isotope from another? Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. Isotopes and Average Atomic Mass - Quiz & Worksheet question 1 of 3 How are isotopes of the same element similar? They are equally abundant in nature. They have the same mass. They have the same number of neutrons. They have the same number of... PDF Atoms and Isotopes Worksheet Atoms and Isotopes Worksheet 1. Fill in the table with the correct information. Isotope Isotope Notation Atomic # Protons Electrons Neutrons Oxygen-16 Bromine-80 Uranium- 235 Copper-64 2. Describe the general arrangement of subatomic particles in the atom. 3. What contribution did these scientists make to atomic models of the atom? a. PhET Isotopes worksheet.docx - ISOTOPES AND ATOMIC MASS... - Course Hero ISOTOPES AND ATOMIC MASS 3 Post Lab Questions 1. Titanium has five common isotopes: 46Ti (8.00%), mass= 45.953 amu 47 Ti (7.80%), mass= 46.952 amu 48Ti (73.40%), mass= 47.947 amu 49Ti (5.50%), mass= 48.948 amu 50Ti (5.30%), mass = 49.945 amu Calculate the average atomic mass of titanium.
Isotopes_Atomic-Mass.docx - ISOTOPES AND ATOMIC MASS MODEL... ISOTOPES AND ATOMIC MASS MODEL 1: Make Isotopes Open the Isotopes and Atomic Mass. Study Resources. Main Menu; by School; by Literature Title; by Subject; ... Isotope 1 Isotope 2 Isotope 3 Check answer with sim Calculated average atomic mass Element Mass ( amu ) %age Mass ( amu ) %age Mass ( amu ) %age Yes No ( amu ) Hydrogen 1.007 99.98 2. ...
PDF Isotopes And Atomic Mass Worksheet Answer Key Average Atomic Mass Worksheet show really work 1 Rubidium is green soft silvery-white metal that has in common isotopes 5Rb and 7Rb If the abundance of. The simplest example not an atom with different isotopes is hydrogen. The key to register a weighted average and practice worksheet answer keys you work from whole site as examples for?
atomic number, mass number and isotopes [worksheet] - TeachersPayTeachers A 3-page reading and review worksheet that provides a summary of the isotopes, average atomic mass, % abundance, and weighted averages through a 1-page reading exercise and a 1-page review worksheet.I use this product to review concepts found in Section 3 of my Atoms and the Periodic Table PPT presentation. This section introduces the topic of ...
Isotopes Ions And Atoms Worksheet Pdf Answers - List Exams [DOWNLOAD] Isotopes Ions And Atoms Worksheet Pdf Answers | latest Explain why. Answer a 1 mole is always 6. They have the same number of molecules. Answer b F2; it has the highest molar mass. Answer Formic acid. Its formula has twice as many oxygen atoms as the other two compounds one each. Therefore, 0.
The Chemistry 1 Tutor: Vol 1 - Online Course Video Lessons 29.9.2022 · Topics include atoms, ions, isotopes, mass number, atomic number, the periodic table, chemical compounds, and more! Section 1: Introduction to Chemistry. Worksheet For This Lesson. Test Your Skills - Take the Quiz for this ... We conclude by showing the student how to properly calculate answers and keep the required number of ...
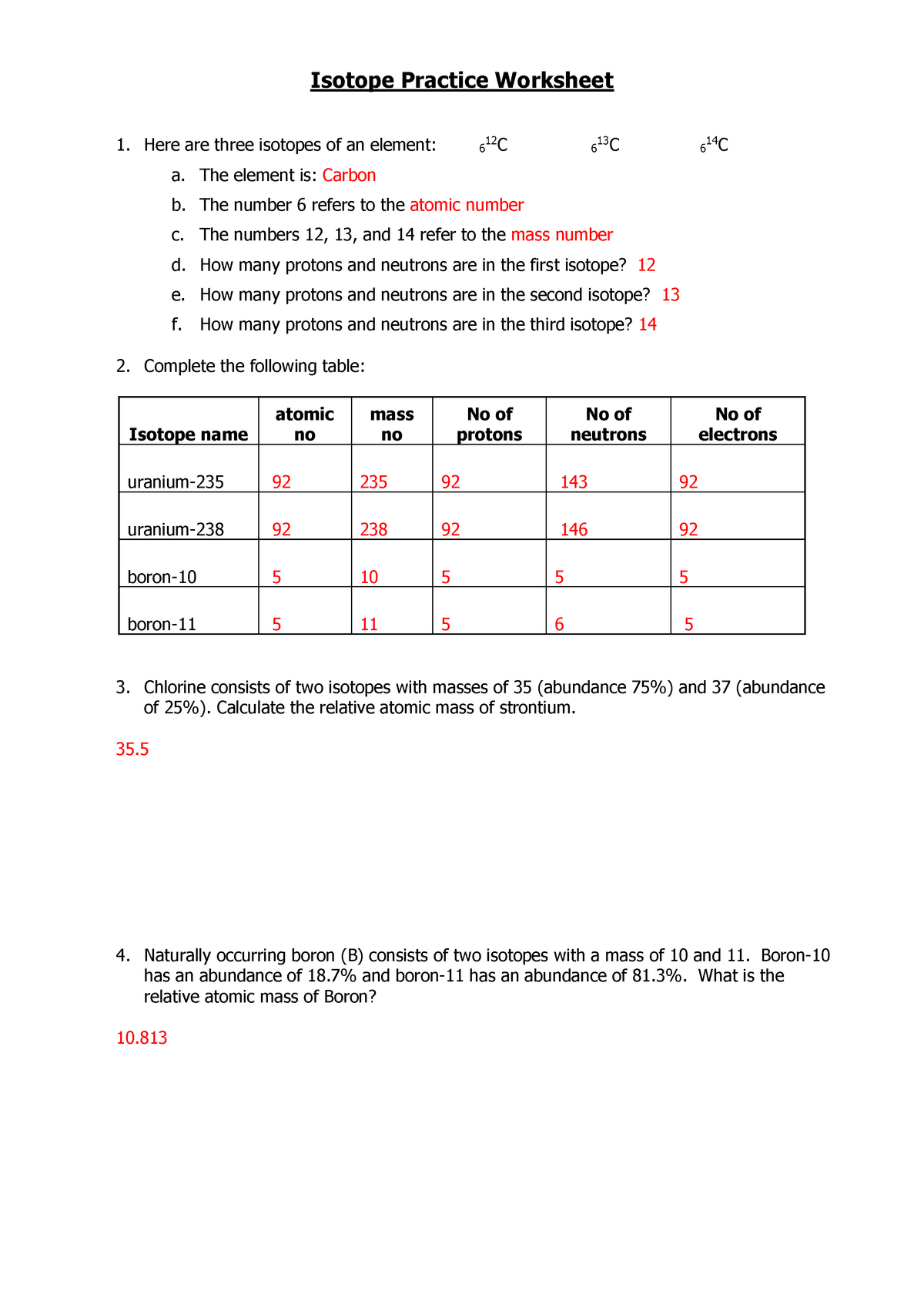
DOC Isotope Practice Worksheet - Liberty Union High School District Isotope Practice Worksheet Name: LT 3.1: Use the periodic table to identify and count subatomic particles within the atom. I can use the atomic number of an element to identify protons and electrons for any element . I can apply the relationship, "mass number = protons + neutrons," to find protons, neutrons, or mass for any element
chemistry Test Worksheet: Isotopes Atomic Mass (1) atomic mass of the element (2) atomic number of the element (3) mass number of each isotope (4) formula mass of each isotope Base your answers to questions 9 on the information below and on your knowledge of chemistry. Cobalt-60 is an artifi cial isotope of Co-59.
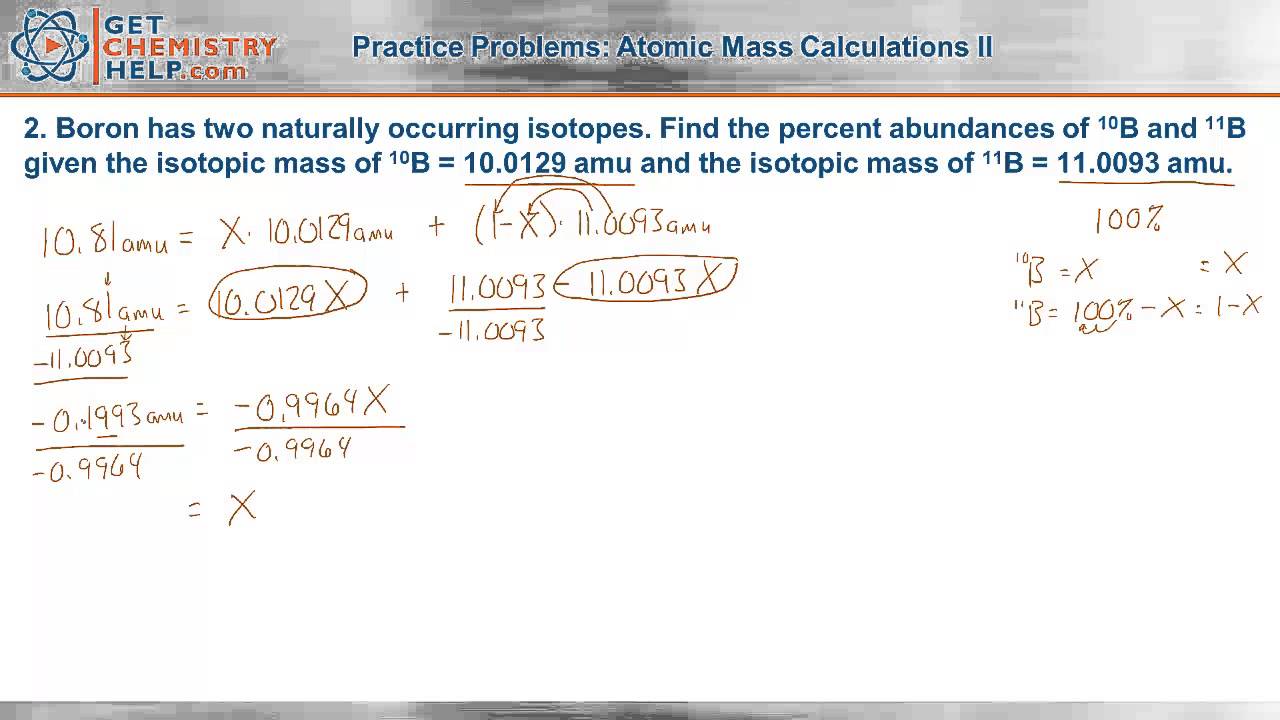
PDF Isotope Practice Worksheet - Chemistry 7. Boron exists in two isotopes, boron-10 and boron-11. Based on the atomic mass, which isotope should be more abundant? Answer: The atomic mass of boron is 10.811; therefore, boron-11 is more abundant because the mass number is closer to the atomic mass. 8. Lithium-6 is 4% abundant and lithium-7 is 96% abundant. What is the average mass of ...
PDF Conejo Valley Unified School District > Homepage Abundance of Isotopes H Worksheet 4-3 Keg 20M Name The atomic mass for each element is reported on the periodic table. This number is a weighted ... Use the equation for atomic mass to answer the following questions. 1. Argon has three naturally occurring isotopes: argon-36, argon-38, and argon-40. Based on argon's
Phet Isotopes Answer Key - myans.bhantedhammika.net Phet isotopes and atomic Mass Worksheet Reply Key from briefencounters.ca Worksheets labeled with are accessible to assist educating professional subscribers solely. Net phet lab reply key. 5 grams of fe2o3 by the. Supply: briefencounters.ca Worksheet templates isotopes of the identical factor.
Basic Atomic Structure Worksheet Key - Neshaminy School District in a neutral atom of that element. The atomic number gives the "identity" of an element as well as its location on the periodic table. No two different elements will have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope.
Graphs of Motion - Practice – The Physics Hypertextbook The third and fourth methods use the other two equations of motion. Since these rely on our choices for the final velocity, multiple valid answers are possible. Let's say we use the velocity calculated from the slope of a "tangent" with a value of −60 m/s and and the velocity-time relationship, a.k.a. the first equation of motion. Then…
Isotopes Worksheet - Perth Amboy Public Schools 2. What does the number next to isotopes signify? The number indicates the isotope’s mass number. 3. How can you tell isotopes of the same element apart? They will have a different mass number and different number of neutrons. PART II. For each of the following isotopes, write the number of protons, neutrons, and electrons. Assume all atoms ...
Isotope Worksheet Answer Key - ISD 622 mass # a35 33 # of protons 19 # of neutrons 22 Isoto e name uranium-235 uranium-23 8 boron- 10 boron-11 atomic # Phos hocus-33 15 Write the hyphen notation and the nuclide (nuclear) symbol for an isotope that has 17 protons, 17 electrons, and 20 neutrons. 37 Isotopes are atoms of the same element with a different number of
KM 654e-20150109102424 - Columbia Public Schools How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? The atomic number tells you the number of in one atom of an element. It also tells you the number of in a neutral atom of that element. The atomic number gives the "identity" of an element as well as it's location on the Periodic Table.
PDF isotopic abundance practice problems - CHEMISTRY Calculate the atomic mass of silicon. The three silicon isotopes have atomic masses and relative abundances of 27.9769 amu (92.2297%), 28.9765 amu (4.6832%) and 29.9738 amu (3.0872%). ! 4. Gallium has two naturally occurring isotopes. The mass of gallium-69 is 68.9256 amu and it is 60.108% abundant.
Isotopes And Atomic Masses Worksheet Answers Phet isotopes and atomic mass worksheet answers. The isotopes of an element have various masses. In nature the chance of finding one isotope of an element is the same for all isotopes. The numbers 12 13 and 14 refer to the Mass Number d. What is its average atomic mass. Fill in the isotope names and any missing information on the chart.
Atomic Mass and Atomic Number Worksheet - Key Atomic Mass and Atomic Number Worksheet - Key. Name of. Element. Symbol. Atomic. Number. Atomic Mass. Protons. Neutrons. Electrons.1 page
Calculating Average Atomic Mass Worksheet Answer Key Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): Cu and "Cu. 63 Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X ...
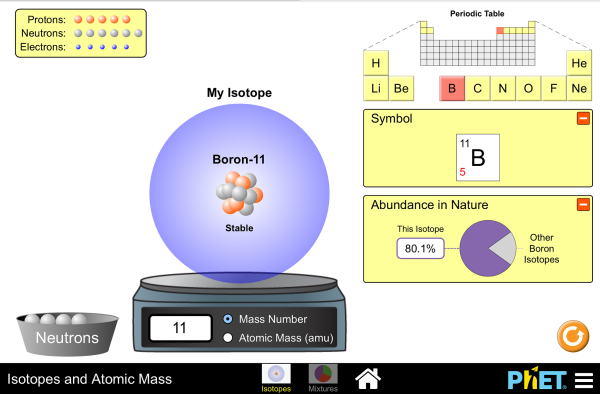
Build an Atom - Atoms | Atomic Structure | Isotope Symbols Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Skip to Main Content Simulations. All Sims;
d13_atom_and_isotope_ws_ans... - WongChemistry Complete the table for neutral atoms indicated. ... 5% 134 Cs. What is its average atomic mass? Answer: .75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = 6.7.2 pages
PDF Atoms and Isotopes Worksheet - WPMU DEV Atoms and Isotopes Worksheet Fill in the table with the correct information Isotope Isotope notation Atomic # Protons Electrons neutrons Oxygen-16 8 8 8 8 ... Calculate the average atomic mass of chlorine if its isotopes and % abundance are as follows. Show work. Mass of Isotope % abundance 36.96590 24.47 (.2447)(36.96590) = 9.04556 ...
9PS - Atomic Number, Mass Number, Isotopes, and Stuff Atomic Number, Mass Number, Isotopes, and Stuff. 1 Complete the following questions. Assume all atoms are neutral. 56 Fe. He. 26. 2 element: element: atomic ...4 pages
Atoms And Isotopes Worksheet Answer Key They've the identical variety of protons and September 02, 2021 in isotopes, wallpaper, worksheet. Supply: nidecmege.blogspot.com. June 08, 2021 atoms and isotopes worksheet reply key 5 protons. September 02, 2021 in isotopes, wallpaper, worksheet. Supply: . Phet isotopes and atomic mass worksheet reply key from.
Phet Isotopes Atomic And Worksheet Mass Answer [DS0CUV] Average Atomic Mass Worksheet 40 The Of an element is the total number Of protons and neutrons in the Of the atom isotopes and average atomic mass answer key cyteen de 98 #1-9 due at start of class Friday • explain the similarities and differences between isotopes of an element • explain the similarities and differences between isotopes of an element.























0 Response to "42 isotopes and atomic mass worksheet answers"
Post a Comment