45 atomic theory review worksheet answers
PDF AP CHEMISTRY REVIEW WORKSHEET (Unit 13 Atomic Structure and Periodicity) Answer the following problems about gases. a. The average atomic mass of naturally occurring neon is 20.18 amu. There are two common isotopes of naturally occurring neon as indicated in the table below. Isotope Mass (amu) % Abundance Ne-20 19.99 Ne-22 21.99 i. Using the information above, calculate the percent abundance of each isotope. ii. Scatter Plot Worksheet With Answers Sep 27, 2021 · For many up-dates and recent information about Scatter Plot Worksheet With Answers pics, please kindly follow us on twitter, path, Instagram and google plus, or you mark this page on book mark area, We attempt to offer you up grade periodically with all new and fresh pictures, enjoy your browsing, and find the best for you.
PDF Answers to Review Questions for Atomic Theory Quiz #1 Answers to Review Questions for Atomic Theory Quiz #1 Multiple Choice Questions: 1. c 7. a 13. c 19. a 25. b 31. b 37. a 43. d 2. d 8. c 14. c 20. c 26. d 32. c 38. d 44. b 3. b 9. a 15. b 21. c 27. b 33. a 39. b 45. a 4. b 10. c 16. c 22. d 28. c 34. c 40. a 46. a 5. d 11. b 17. d 23. d 29. a 35. b 41. a 47.

Atomic theory review worksheet answers
Chemistry 11 Answer Key - Vancouver School Board Atomic Theory Worksheet No 2 (Atomic Theory Worksheet 2 Answer Key.pdf) Atomic Theory Worksheet No 3 (Atomic Theory Worksheet No 3.pdf) Atomic Theory Worksheet No 4 ... Atomic Theory Unit Review Worksheet (U6_RevWS_Key.pdf) Periodic Table. Chapter 11 Worksheet (Chapter 11 Worksheet.pdf) Waves Review - Answers #1 - Physics Classroom Answer: A. In longitudinal waves, particles of the medium vibrate to and from in a direction parallel to the direction of energy transport. If energy is transmitted along a medium from the east end to the west end, then particles of the medium would vibrate eastward and westward PDF Skills Worksheet Directed Reading A - westerville.k12.oh.us In 1911, Rutherford revised the atomic theory. Which of the following is NOT part of that theory? a. Most of the atom's mass is in its nucleus. b. The nucleus is a tiny, dense, positively charged region. c. Positively charged particles that pass close by the nucleus are pushed away by the positive charges in the nucleus. d.
Atomic theory review worksheet answers. Unit 2: Atomic Theory - Ms. Motohashi's Classroom Website Checkpoint 1A , 1AA. Atomic Structure Worksheet & Answers. Atomic Theory II. Concepts Covered: Electronic Structure. Electron Configuration. Core Notation. EC Relationship to Periodic Table. Electron Configuration of Ions. PDF Atomic Theory - Mr. Krohn 8th grade science - Home Development of the Atomic Theory continued What Was the First Scientific Theory of Atoms? The first scientific theory about atoms was published by John Dalton in 1803. Unlike Democritus, Dalton based his ideas on experiments. His theory helped explain observations that he and other scientists had made about elements and compounds. PDF Unit 2 Atomic Theory and Periodicity Review - WPMU DEV His theory of atomic structure led to the "plum pudding" model of atoms Thomson Electron paths cannot be defined for certain Schroedinger and Heisenberg All substances are made of atoms Dalton Section II: Atomic Vocabulary (unscramble) 1. Weighted average of all naturally occurring isotopes of the same element. (mictoa sams) atomic mass 2. Atomic Structure Worksheet Answers - Columbia Public Schools How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? The atomic number tells you the number of in one atom of an element. It also tells you the number of in a neutral atom of that element. The atomic number gives the "identity" of an element as well as it's location on the Periodic Table.
Chapter 1 Structure and Bonding - Michigan State University The atomic mass (atomic weight) of an element is weighted average mass in atomic mass units (amu) of an element’s naturally occurring isotopes. Carbon: Atomic Number and Atomic Mass 12C 6 AC Z 13C 6 (98.9 12.000) (1.1 13.000) 12.011 100 × +× = PDF Questions for Atomic Theory Quiz #1 - Patterson Science Dalton: his atomic theory, Law of Conservation of Mass, Law of Constant Composition Atom Read Handout: In Search of a Model for Matter Read pages 23 to 25 in your text Answer questions 1 to 5 on Handout: Practice Questions: The Development of the Modern ic Theory 2 History of Atomic Theory (cont.) of the atomic models PDF Skills Worksheet Concept Review - whsd.net Concept Review: Energy 1. energy 2. physical 3. chemical 4. endothermic 5. exothermic 6. kinetic 7. transferred 8. In any chemical or physical change, the total quantity of energy remains constant. Energy cannot be created or destroyed. 9. Heat is the enegy transferred between objects that are at different tempera- tures. 10. Atomic Theory and Structure Review worksheet Atomic Theory and Structure ReviewAtomic Theory and Structure. ID: 2835540. Language: English. School subject: Chemistry. Grade/level: 11. Age: 16-18. Main content: Atomic Theory and Structure. Other contents: Unit Test Review. Add to my workbooks (0)
Atomic Nucleus: Definition, Structure & Size - Study.com Oct 12, 2021 · The atomic nucleus is the center of an atom, which holds most of the atom's mass as well as its neutrons and protons. Explore the definition, structure, and size of the atomic nucleus. PDF Chemistry Test #2: Atomic Theory and the Periodic Table (KEY!) The review is divided into sections for a REASON. GO BACK to your NOTES!!! 1. Using the picture above, explain Rutherford's gold foil experiment. What did the gold foil experiment tell us about the structure of the atom? 2. Draw the Bohr model for the following elements (depict protons as + and electrons as -): Fluorine Neon Atomic Structure Test Review Worksheet - bossinspire Basic atomic structure worksheet answers 1 a protons b neutrons c electrons a positive b neutral c negative 2 atomic number or identity. Chem a unit 3 atomic structure test review please complete on a. Atomic structure review worksheet answer key along with supportive issues. Start studying atomic structure test review. DOC Chapter 4 Review Worksheet - Currituck County Schools 1. Complete the following table. Element Symbol Number of Protons Number of electrons Number of neutrons Atomic Number Mass Number 25 53 11 12 35 45 39 89 33 75 Ac 227 2. Fill in the following Table Element Symbol Atomic Number Mass Number Number of neutrons nitrogen-15 8 Beryllium-9 4 3.
Waves Review - Answers - Physics Classroom Answer: E. A, B, and C can be quickly ruled out since it shows the amplitude of the reflected and incident pulse to be the same size. An incident pulse would give up some of its energy to the transmitted pulse at the boundary, thus making the amplitude of the reflected pulse less than that of the incident pulse.
PDF Atomic Structure Review Worksheet Key - Springtown ISD Atomic Structure Review Worksheet Key 1. 12 found below symbol on periodic table 2. Na atomic number 11 is Sodium 3. 4 mass - protons 7-3 = 4 4. 10 protons = electrons 5. 5 found by looking for the atomic number ... True 0 atomic mass units for an electron 1 atomic mass unit for protons and neutrons 15. True The electron cloud is mainly empty ...
Atomic Structure TEST REVIEW Flashcards - Quizlet 24.287. Uranium is used in nuclear reactors and is a rare element on earth. Uranium has three common isotopes. Using the chart below determine the average atomic mass of Uranium. Submit your answer with three decimal places. Isotope Mass Percent. Uranium-234 234.097 0.01. Uranium-235 235.013 0.71. Uranium-238 238.047 99.28.
Atomic Theory Worksheets | Teachers Pay Teachers This worksheet contains basic conceptual questions about History of Atomic Theory.What's included in this resource?Printable and editable Student Worksheet (PDF and Word document)Paperless digital version for use in Google Drive (Prepared with Google Slides)Complete Answer KeyFor updates about sales Subjects: Chemistry, General Science, Science
DOC Chemistry Webquest #1: Introduction to Atoms Worksheet In 1909 this scientist demonstrated that the atom is mostly empty space with a small positively charged nucleus containing most of the mass and low mass negatively charged particles orbiting this nucleus. What date did Neils Bohr developed the first successful model of the atom? History of the Atom Timeline 1 2 3 4 5 6 Date: 400 BC 1928
Atomic Theory Discussion worksheet 2.02 answer key.pdf Answer choice g is more of a silly choice and is just incorrect. 2.
Quiz & Worksheet - Atomic Theory | Study.com Worksheet. 1. The basic, modern concept of atomic theory did not develop until: The 7th century B.C. The 19th century. The 17th century. The acceptance of the Heisenberg principle. 2. Philosophers ...
Atomic Theory - Weebly Lecture Atomic Theory. Lecture note taking outline. Conservation of Matter Lab. Atomic Structure Worksheet. Phet Simulation Build an Atom activity. Atomic Theory Worksheet Packet. Atomic Structure Practice. Atomic Theory Unit Review.
Honors Chemistry Worksheet - Atomic Structure - Quia What is the modern atomic theory? The atom possesses a very small, very dense, positively charged core called the nucleus. It is composed of nucleons, the major ones being protons and neutrons. The electrons travel randomly around the nucleus in energetically permissible regions which make up the electron cloud. 2.
PDF The Atom for Middle School - Miss Little's Classroom Website Write the letter for the atomic model in front of the statement. 1. _____ Atoms are indivisible. 2. _____ An atom is the smallest piece of matter. 3. _____ In an atom, electrons are located in energy levels that are a certain distance from the nucleus. 4. _____ Atoms are small, hard particles. 5.
PDF CHAPTER 6 Elements, Atoms, and the Atomic Theory - Miss S. Harvey Atomic Theory CHAPTER Matter is made of atoms. Atoms have a structure that determines their properties. The Periodic Table organizes elements in different ways. 148 NEL Chapter Preview Matter has mass and takes up space. It is the ÒstuffÓ that makes up the universe: the stars, the planets, the atmosphere, the rocks, our food, our toys, and ...
Chadwick Atomic Model | James Chadwick Atomic Theory ... Nov 17, 2021 · Learn the theory of James Chadwick, his experiment, and his contribution to atomic theory, including his discovery. See the atomic model of James Chadwick. Updated: 11/17/2021
PDF Atomic Structure Review Worksheet - Springtown ISD Atomic Structure Review Worksheet Ions 21. What is the charge of an atom with 7 protons and 9 electrons: _____ 22. What is the charge on an ion with 3 protons and 1 electron: _____ 23. What is the charge on an ion a Chlorine ion with 17 protons and 18 electrons: _____ 24. What is the charge if there are 59 protons and 62 electrons: _____ ...
PDF ATOMIC THEORY - SAMPLE TEST QUESTIONS - Key C.'Bohr'Diagrams'and'Energy'Level'Diagrams' 1.'Describe'the'three'rules'to'be'used'for'determining'electron'assignment:'
Mr Johnson's Chemistry - ch4 atomic theory review answers List the 5 atomic models we studied. Make a drawing of each. historical order: d, t, r,b, q Match these statements with a model gold foil - r cathode ray - t spectrum - b cloud - q uncertain -q...


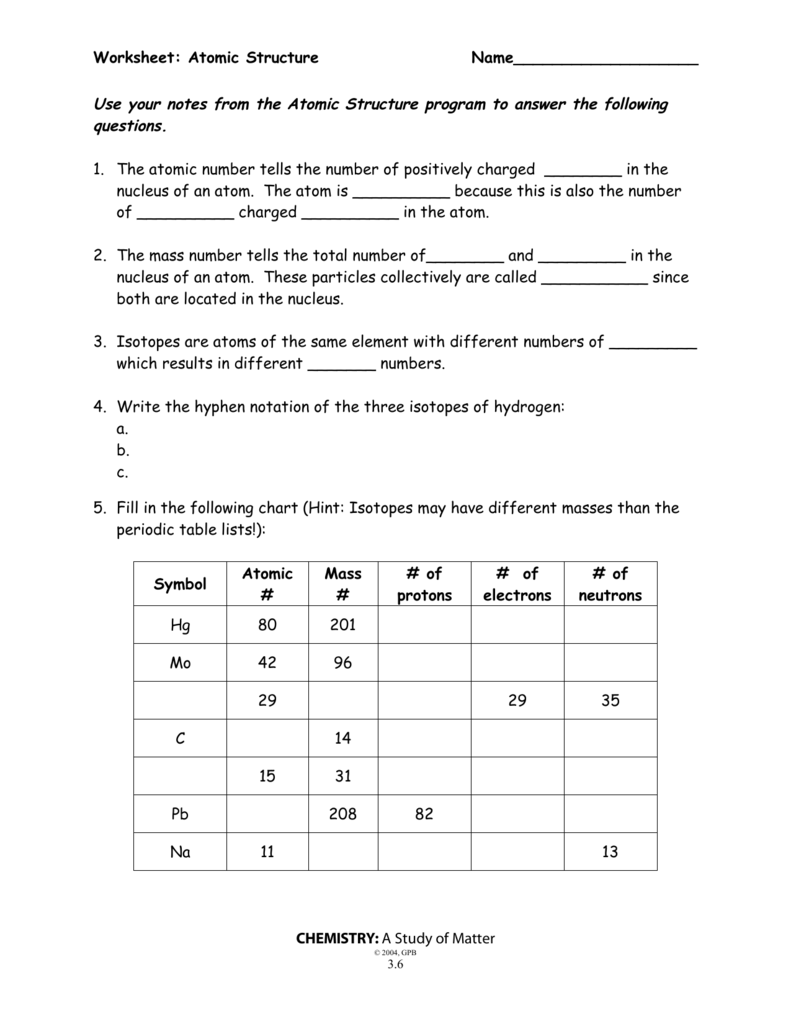








0 Response to "45 atomic theory review worksheet answers"
Post a Comment