42 chemistry ef and mf worksheet answers
DOC % Composition, Empirical Formula and Molecular Formula Worksheet - Weebly EF = PdH2. Multiple = 2. MF = Pd2H4. Octane, a compound of hydrogen and carbon, has a molar mass of 114.26 g/mol. If one mole of the compound contains 18.17 g of hydrogen, what is its molecular formula? C8H18. Find the molecular formula of a compound that contains 30.45 % nitrogen and 69.55 % oxygen. The molar mass of the compound is 92.02 g ... PDF Honors Chemistry Name - mcvts.net Complete these problems on a separate sheet of paper. 1. Determine the percent Oxygen in Potassium Perchlorate. KClO4 Molar Mass is 138.547 g KClO4 4 moles of O in KClO4 Molar mass of O is 15.999 4 moles O = 63.996 g !"#$"%&!!"#$"%&'&"(!!"!!=! 63.996!!! 138.547!! !!!100=!46.19!% 2. Determine the percent Aluminum in Aluminum Sulfate. Al2(SO4)3
Bill Nye Chemical Reactions Worksheet Answers - Organicard Water is two parts h and one part o. Answers to practice problems 1. The second is a fill in the blank version with optional word bank. Worksheet 7th grade bill nye chemical reactions worksheet answers january 16, 2022january 16, 2022 play this game to review chemical reactions. Bill nye chemical reactions worksheet.

Chemistry ef and mf worksheet answers
Chapter 7 - Edison Honors Chemistry - Google Homework #1, skip question 12 correction: #20 answer Cu3N2 ... Determining Molecular Formulas Worksheet.pdf View: Homework #5 Dec 5, 2017, 9:59 AM: edison honors chemistry: ċ. EF_MF from Combustion Data.docx View: ... percent composition and EF worksheet.pdf View: Homework#4 ... worksheet_-_empirical_and_molecular_formula_answers... - Course Hero The empirical formula is the lowest whole number ratio of atoms in a compound . The molecular formula shows the true number of atoms in the molecule and is a whole number multiple of the empirical formula . EF and MF worksheet.doc - Course Hero EMPIRICAL FORMULA & MOLECULAR FORMULA WORKSHEET 1. Find the molecular formula of a compound that has a molar mass of 26 g/mol, and has an empirical formula of CH. EF is CH, mass = 12 + 1 = 13 g/mol MF = n (EF) n = molar mass ÷ empirical mass, = 26 ÷ 13 = 2. So MF = C 2 H 2 2.
Chemistry ef and mf worksheet answers. PDF Ms. Demonte's Chemistry Classes - Home be down famuja_ Representing # of EF units in each MF CH Molecular Formula Model 2: Determining Molecular Formulas Substance Name Methane Eth ne Benzene Ethene Pro ene C clohexane Ethane Molecular Mass @ (MF mass) 16.0 26.0 78.0 28.0 42.0 84.0 30.0 Empirical formula CH CH CH CH CH CH CH Empirical formula mass (g) 13.0 14.0 DOC template 4. A compound composed of hydrogen and oxygen is found to contain 0.59 g of hydrogen and 9.40 g of oxygen. The molar mass of this compound is 34.0 g/mol. Find the empirical and molecular formulas. 5. A sample of iron oxide was found to contain 1.116 g of iron and 0.480 g of oxygen. Its molar mass is roughly 5 x as great as that of oxygen gas, O2. Homework Assignments - Mr Z's Chemistry - Google "Percent Composition And Empirical Formula Worksheet 7-3 (PC, EF & MF).pdf" Sections 15, 02 and 21: Review for your exam on Chapter 7. Answers to the review sheets are at the bottom of this page. "Mole Mega Worksheet (Master).pdf" and "Molecular Formula Worksheet (Master).pdf PDF Georgetown Independent School District / GISD Home Find the empirical formula of a compound that contains 15.8 g of Al, 28.1 g of S, and 56.1 g of oxygen. (Answer: A12S3012 or 40. The empirical formula for a hydrocarbon is CH2. Its actual molar mass is 294 g/mol. What is its molecular formula?
LibGuides: IB Chemistry: Chapter 1- Formulas, Reactions, Moles ... Formulas and Reactions Reactions #1 Diatomic Molecules Reactions #2 Balancing Equations Reactions #3 Types of Reactions Intro Reactions #4 Types of Reactions detail Reactions #5-Predicting Products Chemical Reactions Package Chemical Reactions Package Answers 4. Moles and Density Moles and Density Moles and Density worksheet with answers Cool Higher Chemistry Calculations Questions And Answers 2022 The ap chemistry exam assesses your ability to understand science practices in relation to four big ideas: The difference between an atom and an element is a. Chemistry Ef And Mf Worksheet Answers Promotiontablecovers from promotiontablecovers.blogspot.com Atoms are held together by. An element is made of atoms b. Chapter 7.3 & 7.4 Formula Analysis | Academic Additional Practice with Answers ... EF ws key pkt. MF ws key. Ch 8 WS 5 Solutions. Individual Lab Scenario Prob. Mole, EF, hydrate worksheet. review guide 7.3 & 7.4. Ch 7 part 2 review key. Nomenclature tutorial.pdf. Tel: 724-941-6250 x 8321 Email: demascalw@pt-sd.org ... Chemistry Flashcards | Quizlet 1.0 kg = 1000g/1kg =6.02210^23molecules/17.04g =3H atoms/ 1 molecule ANSWER: 1.0610^26. Percent Composition %A = parts of A / total parts *100. ... (EF)n, Refer to previous example (EF=MF) C2H4. Empirical formula = CH2 CnH2n ... Chemistry Chapter 10 56 Terms. Kimberly_Samsel. Empirical and Molecular Formula Calculations 17 Terms.
Some basic concepts of chemistry - Class Notes 1)Empirical Formula 2)Molecular mass Step 1 Calculation of the empirical formula from the percentage composition. Step 2 Calculation of empirical formula mass by adding the atomic mass of all the atoms present in the empirical formula. Step 3 Determination of the molecular mass of the compound from the given data. Unit 7 - Chemical Reactions - rocklinusd.org Partial Key- make sure you check your answers! ... Assignment #1 - % composition, Empirical vs. molecular formula, Calculations for EF and MF; Assign #2 - Oxidation/ Reduction Reactions; Worksheets & Notes Worksheets & Notes. Unit 7 PowerPoint Slides; Language of Chemistry Lab; Chemical vs. Physical Reactions; PDF CHEMISTRY CP Name: KEY Period: - oakparkusd.org Find the empirical formula of a compound which is 49.5% Fe, and 50.5% F. ♦Step 1:Convert to grams Fe: 49.5 grams F: 50.5 grams Step 2:Convert grams to Moles Fe: 49.5 grams = 0.887 M F: 50.5 grams = 2.66 M 55.8 grams 19.0 grams Step 3:Divide by Smallest Moles Fe: 0.887M = 1 F: 2.66M = 3 EF: FeF 3 PDF Livingston Public Schools / LPS Homepage Worksheet 1 1.2 Mole Conversions Worksheet There are three mole equalities: 23 1 mol 6.02 x 10 particles 1 mol = molar mass 1 mol = 22.4 L (for a gas at STP) Mole-Particle Conversions 1. 1. 2. 3. 4. 5. 22 How many moles of magnesium is equivalent to 3.01 x 10 atoms of magnesium? 3. 01 * (O 0.0500 mol MB
PDF Unit V. Review Worksheet Answers. - LPS a. EF= CH 3 MF= C 2H 6 b. EF= NO 2 MF= N 2O 4. c.EF= C 4H 5N 2O MF= C 8H 10N 4O 2 d. EF= NH 2Cl MF=NH 2Cl . 10. Using the UNBALANCED chemical equation: Ga + HCl GaCl 3 + H 2. Solve for the following ** Assume excess of the other reactant . a. The moles of Gallium chloride that are produced from 2.5 moles of Ga b.
SCH3UI Unit 3 Chemical Quantities - Mr. Arthur's Science Page AP Chemistry Contact Me SCH3UI Unit 2 Naming & Reactions Mr. Arthur's Science Page ... percentage_composition_worksheet_answers.pdf: File Size: 15 kb: File Type: pdf: Download File _composition_answers.pdf: ... ef__mf_answers.pdf: File Size: 215 kb: File Type: pdf: Download File. unit_3_day_9__2.pdf: File Size: 189 kb:
Molecular Formula Practice Test Questions - ThoughtCo Answers appear after the final question. Question 1 An unknown compound is found to contain 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen with a molecular mass of 60.0 g/mol. What is the molecular formula of the unknown compound? Question 2 A hydrocarbon is a compound comprised of carbon and hydrogen atoms.
Empirical & Molecular Formulas Quiz - Softschools.com To get the molecular formula, you must divide the molar mass of the empirical formula into the given molecular formula mass to find the multiplier. Then multiply that number by the EF to get the MF. To complete this quiz, you must have a periodic table and a calculator. This quiz covers simple empirical and molecular formula calculations.
factor label worksheet Chemistry Ef And Mf Worksheet Answers - Promotiontablecovers promotiontablecovers.blogspot.com. mole moles conversions stoichiometry molar calculations atomic mf mishkanet askworksheet exploration bashahighschoolband chartsheet. Business Plan textbook.stpauls.br.
PDF Determination of Empirical and Molecular Formulas E.F ... - Cornell College Determination of Empirical and Molecular Formulas Empirical Formula, E.F. (atom ratio): from % composition by weight. Substance contains 40.0% carbon, 6.7% hydrogen, (assume remainder, 53.3% is oxygen).
PDF Answer KEY - CSI : Case Profile Tony DeMoy Answer KEY - CSI : Case Profile - ... 151g/mol EF: C 8 H 9 NO 2 MF: C 8 H 9 NO 2 Compound Name: Paracetamol ... Based on your findings, you conclude that the murderer was Finley Finch. Title: EMPIRICAL FORMULA WORKSHEET Author: J. David Hill Created Date: 2/4/2021 2:56:20 PM ...
3.2 Determining Empirical and Molecular Formulas - Chemistry 2e - OpenStax This same approach may be taken considering a pair of molecules, a dozen molecules, or a mole of molecules, etc. The latter amount is most convenient and would simply involve the use of molar masses instead of atomic and formula masses, as demonstrated Example 3.10.As long as the molecular or empirical formula of the compound in question is known, the percent composition may be derived from ...
PDF Part 2 and 3 HW Key and Notes - Conejo Valley Unified School District below. Show your work and use this data to answer the following: Mass of empty crucible 25.83 g Mass of crucible and hydrate 30.83 g Mass of crucible and compound after 1st heating = 27.68 g. Mass of crucible and compound after 2nd heating 27.68 g. 11. Determine the mass of the hydrate before heating. 12. Determine the mass of the anhydrous ...
PDF 11. Empirical Formula Worksheet II EMPIRICAL AND MOLECULAR FORMULA WORKSHEET 1. An oxide of chromium is found to have the following % composition: 68.4 % Cr and 31.6 % O. Determine this compound's empirical fomula. I $15 CX2Ô3 2. The percent composition of a compound was found to be 63.5 % silver, 8.2 % nitrogen, and 28.3 % oxygen. Determine the compound's empirical formula. 3.
PDF Example: EMPIRICAL FORMULA - chemunlimited.com CHEMISTRY E.F. AND M. F. Worksheet Cd0.434C1.67H2.62O1.74. These whole numbers become the subscripts for the empirical formula: CdC4H6O4 . ... You can see from this example that the MF is a multiple of the EF and represents the actual number ... CHEMISTRY E.F. AND M. F. Worksheet. 3. An unknown compound is analyzed and found to consist of 24.3 ...
EF and MF worksheet.doc - Course Hero EMPIRICAL FORMULA & MOLECULAR FORMULA WORKSHEET 1. Find the molecular formula of a compound that has a molar mass of 26 g/mol, and has an empirical formula of CH. EF is CH, mass = 12 + 1 = 13 g/mol MF = n (EF) n = molar mass ÷ empirical mass, = 26 ÷ 13 = 2. So MF = C 2 H 2 2.
worksheet_-_empirical_and_molecular_formula_answers... - Course Hero The empirical formula is the lowest whole number ratio of atoms in a compound . The molecular formula shows the true number of atoms in the molecule and is a whole number multiple of the empirical formula .
Chapter 7 - Edison Honors Chemistry - Google Homework #1, skip question 12 correction: #20 answer Cu3N2 ... Determining Molecular Formulas Worksheet.pdf View: Homework #5 Dec 5, 2017, 9:59 AM: edison honors chemistry: ċ. EF_MF from Combustion Data.docx View: ... percent composition and EF worksheet.pdf View: Homework#4 ...




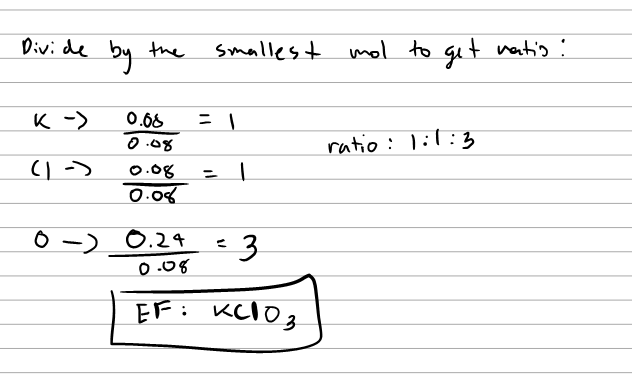






0 Response to "42 chemistry ef and mf worksheet answers"
Post a Comment