41 conjugate acid base pairs worksheet answers
Worksheet # 3 Conjugate Acid-Base Pairs Worksheet # 2 Conjugate Acid-Base Pairs. Complete each acid reaction. Worksheet # 4 Using Acid Strength Tables. Acid-base reactions can be considered to be a competition for protons. A stronger acid can cause a weaker acid to act like a base. Conjugate acid - Wikipedia A conjugate acid, within the Brønsted-Lowry acid-base theory, is a chemical compound formed when an acid donates a proton (H+) to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a hydrogen ion.
PDF Conjugate Acid Base Pairs Chem Worksheet 19-2 An acid Acids donate protons acceptor. The substance that is produced after an acid has donated its. proton is called the conjugate base while the substance formed when a. can donate a proton to the conjugate base, to reform the original. A proton is a hydrogen ion reactants in the reverse reaction.

Conjugate acid base pairs worksheet answers
Conjugate Acid Base Pairs Worksheet | Fronteirastral.com Acids and Bases Worksheet Answers from Conjugate Acid Base Pairs Worksheet , source: homeschooldressage.com. PDF Check Your Solution The conjugate acid-base pairs differ by one... Identify the conjugate acid-base pairs in each reaction. The base is the molecule or ion that accepts the proton. On the right side of the equation, you can identify the conjugate acid and base by the difference of a single proton from the base and acid on the left side. Theories of acids and bases | A second example of conjugate pairs It also explains the concept of a conjugate pair - an acid and its conjugate base, or a base and its conjugate acid. The Arrhenius theory is of historical interest only, and you are unlikely to need it unless you are doing some work on the development of ideas in chemistry.
Conjugate acid base pairs worksheet answers. Worksheets - Conjugate Acid/Base Pairs Worksheet. Conjugate Acid/Base Pairs. Determine the conjugate acid for each: H2O. F (DOC) Acid and Base Worksheet -Answers | Sanhot... - Academia.edu Download Free PDF. Acid and Base Worksheet -Answers. Explain this, based on the definitions of acids and bases that we discussed in class. Write the conjugate base for each of the following: a. H2S c. NH3 -1 b. HS d. H2SO3 18. a. HS-1 b. S-2 c. NH2-1 d. HSO3-1 pH practice - Answers 1)... Conjugate Acid Base Pairs Worksheet | Homeschooldressage.com Worksheet Molarity Problems Worksheet Time Study Worksheet from Conjugate Acid Base Pairs Worksheet, source:cathhsli.org. acids & bases bronsted lowry and K acid from Conjugate Acid Base Pairs Worksheet, source:teachinghighschoolchemistry.com. Conjugate acid-base pairs (video) | Khan Academy In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺). A conjugate acid is formed when a proton is added to a base, and a conjugate Conjugate acid-base pairs. This is the currently selected item.
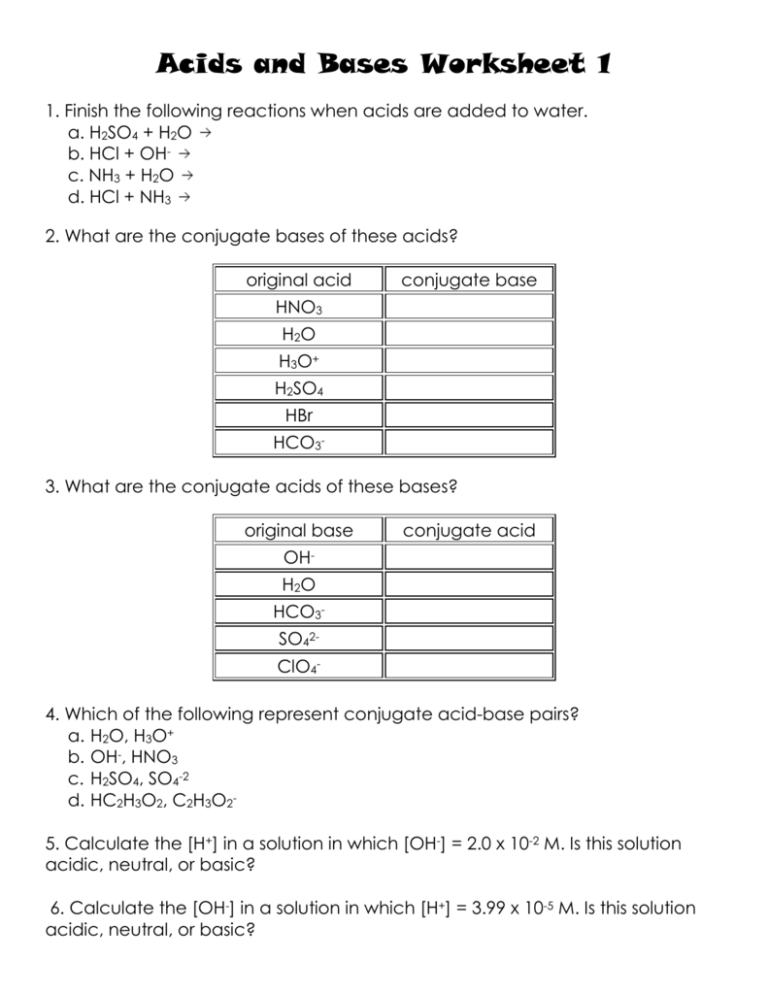
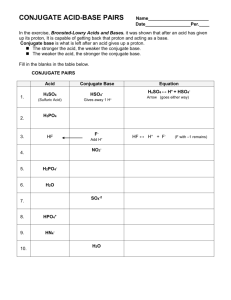
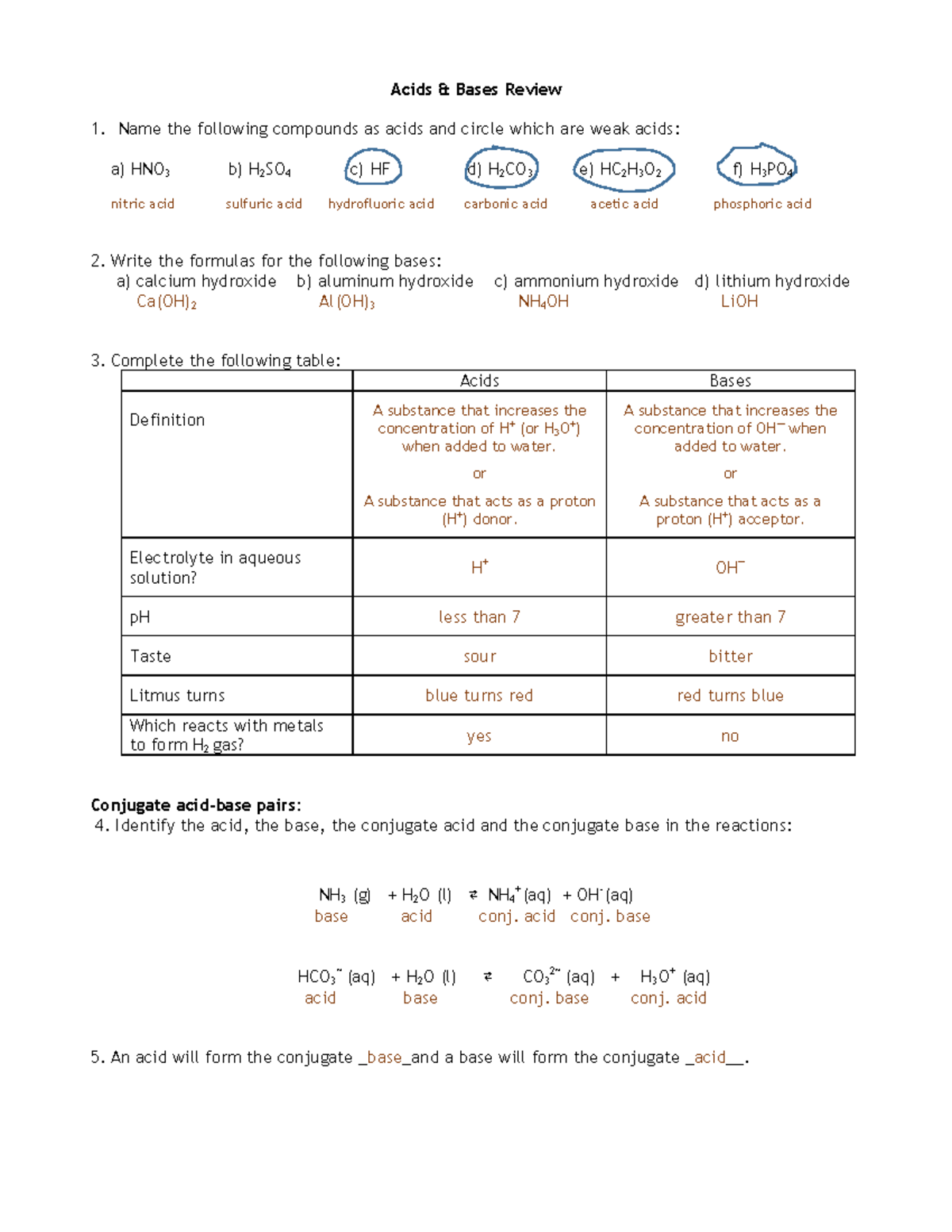
Conjugate Acid-Base Pairs Conjugate Acid-Base Pairs. Now that we've defined acids and bases, let's discuss how they work together in reactions. Apply the appropriate acid-base theory to first identify the acid and base reacting and then identify the conjugate acid-base pairs in the examples below Conjugate Pairs Worksheet - Name: _ Conjugate Pairs... ...Conjugate Pairs Worksheet Identify the acid (A), base (B), conjugate acid (CA), and conjugate base (CB) for each of the following. a) HClO 4 (aq) + H 2 O(l) Worksheet 10 - Conjugate acid base pair.doc. test_prep. 2. conjugate_pairs_practice_w_answers (1).docx. Dulles H S. COMPSCI 111. Conjugate Acid-Base Pairs Acid and base chart lists the strength of acids and bases (strongest to weakest) in order. Simple to use laboratory reference chart for scientists, researchers The strong bases are listed at the bottom-right of the table and get weaker as we move to the top of the table. Conjugate Acid-Base Pairs. Conjugate Acid Base Pairs Worksheet | PDF | Acid | Chemistry Download now. SaveSave Conjugate Acid Base Pairs Worksheet For Later. 100%(3)100% found this document useful (3 votes). 4K views1 page. Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations. 1. HCl + NH3 2. OH + HCN 3. PO43 + HNO3 4. HCO3 + HCl.
Conjugate Acid Base Pairs Worksheet - DocShare.tips acid. base. Acids donate protons Bases accept protons A proton is a hydrogen ion. H3O+ + F-. c. acid. c. base. Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations. 1. HCl + NH3 2. OH- + HCN 3. PO43- + HNO3 4. HCO3- + HCl. Conjugate acid-base pairs | Siyavula Conjugate acid-base pairs (ESBQX). Look at the reaction between hydrochloric acid and ammonia to form ammonium and chloride ions (again we have highlighted the Write a chemical equation for the reaction of these two compounds. Now identify the conjugate acid-base pairs in your chosen reaction. What is a conjugate acid and base pair? + Example The concept of conjugate acid-base pair is related to Bronsted-Lowry acid-base theory and according to this theory, acid is a proton #(H^+)# donor while base is a proton acceptor . Let's focus on the first example, #CH_3COOH#. It behaves as an acid because it donates a proton and becomes... What is a conjugate acid and base pair? Explain with examples. Hence conjugate acid -base pairs differ only in hydrogen ions. (H+). . Complete Step By Step Answer: According to the Bronsted-Lowry acid base theory concept In order to explain a conjugate acid and base pair. Let us consider the following reaction: CH3COOH+H2O→CH3COO−+H3O+.
Conjugate Acid-Base Pairs Sample Problems - YouTube Conjugate Acid-Base Pairs Sample Problems. Смотреть позже. Поделиться.
Conjugate Acid Base Pairs Worksheet Answers Bronsted Lowry Acid Base Pairs The Best Worksheets Image Collection. Conjugate acid base pairs name chem worksheet 19 2 base acid c. Bronsted lowry base definition examples video lesson from conjugate acid base pairs worksheet source.
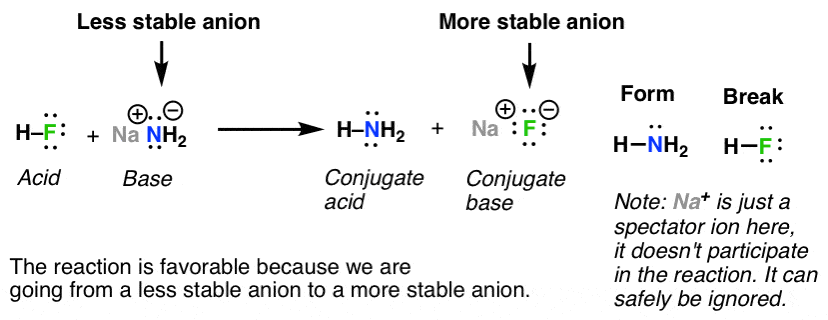
PDF Worksheet 20 - Acids and Bases The Brønsted-Lowry definit base conjugate acid. In this reaction, HF is the species losing the proton (H'), making it the acid. The equilibrium concentrations of these species will be determined by the relative strengths of the acids and bases. The strongest acid will dissociate to the greatest extent.
Acids and Bases Review Worksheet Answers - Acids... - StuDocu Lewis Structures Practice Exercises Answers. Module 2 Practice Solutions. Vsepr Handout. Acids and Bases Review Worksheet. Conjugate acid-base pairs
11.13: Conjugate Acid-Base Pairs - Chemistry LibreTexts The use of conjugate acid-base pairs allows us to make a very simple statement about relative strengths of acids and bases. When the slope of the line is not far from horizontal, the conjugate pairs are not very different in strength, and the reaction goes only part way to completion.
PDF Week 8 Worksheet: Chapter 10 Acids and Bases Acid or Base base base acid base acid base acid acid base base base acid acid. Arrhenius Bronsted-Lowry. Ternary Acids/Bases Explain the order of increasing or decreasing acid strength and conjugate base strength for the. following groups: (a) H2SO3, H2SO4 The more oxygens there...
acid base problems worksheet - Search Conjugate Acid Base Pairs Chem Worksheet 19-2 Name An acid is defined as a proton donor while a base is a proton Acids donate protons acceptor. Lewis acid/base theory (sometimes called donor-acceptor theory) is a broad, widely applicable approach to the classification of chemical substances...
PDF Microsoft Word - 314 acid-base list, answers newer.DOC Acid/Base arrow pushing worksheet. 1. These proton transfer reactions are the first step in multistep mechanisms to be studied later in the course. Supply the necessary curved arrows, lone pairs of electrons and/or formal charge to show how the first step each reaction proceeds.
Acids, bases, and salts worksheet Flashcards | Quizlet What is a conjugate acid pair. It consists of two substances related by the loss or gain of a single hydrogen ion. A substance that can act as an acid and a base is True or false: All the acids and bases included in the Brønsted-Lowry theory are also acids and bases according to the Lewis theory.
Conjugate Acids and Bases (with worksheets, videos, solutions...) • Understanding acid-base conjugate pairs. • Equilibrium Systems Conjugate acid and base equilibrium systems are a type of chemical equilibrium in which the rate of the forward Try the given examples, or type in your own problem and check your answer with the step-by-step explanations.
Conjugate Acid Base Pairs Worksheet - worksheet Conjugate acid base pairs determine the conjugate acid for each. Showing top 8 worksheets in the category chemistry 19 2. B ch3nh2 h2o ch3nh3 oh 1 In both cases identify the conjugate acid base pairs. Po 4 3 hno 3 no 3 hpo 4 2 4. Hcl nh 3 nh 4 cl 2. O is dissolved in water the solution turns basic...
What is conjugate acid base pair? - Quora A conjugate acid-base pair contains two compounds that differ only by a hydrogen ion (H+) and a charge of +1. according to this reaction, there is a reaction between water and ammonia which in this case are the acid and base respectively becaus...
Theories of acids and bases | A second example of conjugate pairs It also explains the concept of a conjugate pair - an acid and its conjugate base, or a base and its conjugate acid. The Arrhenius theory is of historical interest only, and you are unlikely to need it unless you are doing some work on the development of ideas in chemistry.
PDF Check Your Solution The conjugate acid-base pairs differ by one... Identify the conjugate acid-base pairs in each reaction. The base is the molecule or ion that accepts the proton. On the right side of the equation, you can identify the conjugate acid and base by the difference of a single proton from the base and acid on the left side.
Conjugate Acid Base Pairs Worksheet | Fronteirastral.com Acids and Bases Worksheet Answers from Conjugate Acid Base Pairs Worksheet , source: homeschooldressage.com.

























0 Response to "41 conjugate acid base pairs worksheet answers"
Post a Comment