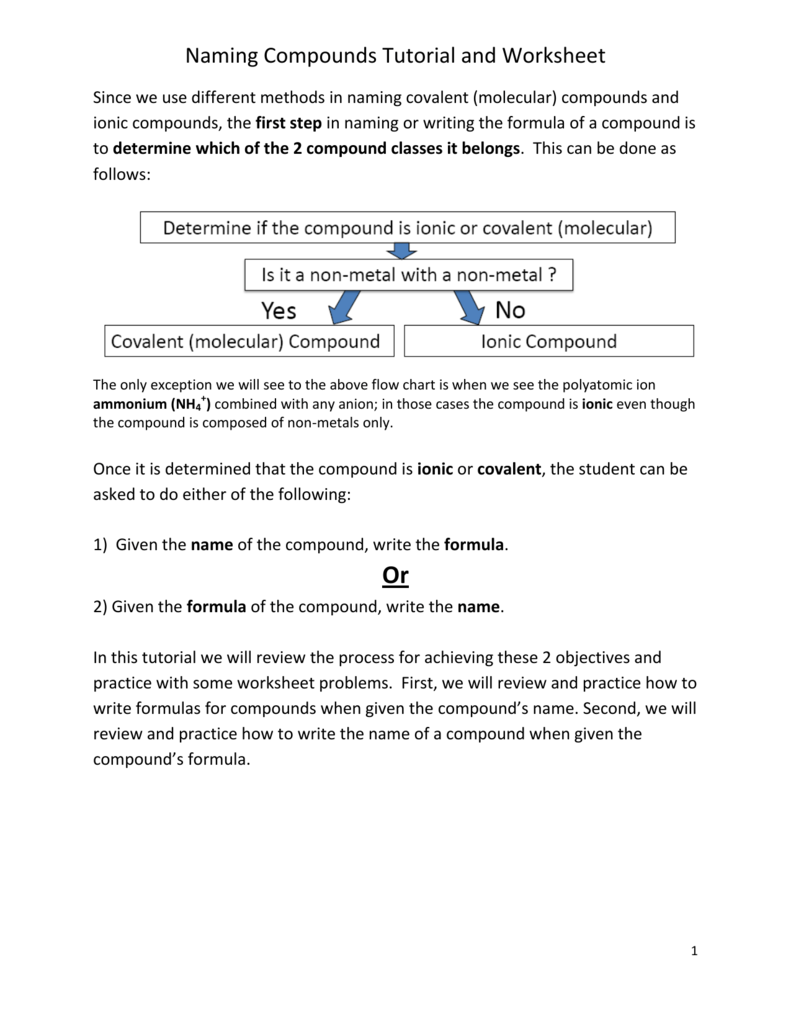
40 covalent compounds worksheet formula writing and naming
covalent compounds. b) concrete: ionic compounds. c) gasoline: covalent compounds. d) candy corn: covalent compounds. 2) Name the following covalent compounds: a) SiF4 . silicon tetrafluoride. b) N2S3 . dinitrogen trisulfide. c) HBr . hydrogen bromide (or hydrobromic acid) d) Br2 . bromine. 3) Write the formulas for the following covalent ... Naming Rules / Worksheet #1 Name _____ Date _____ Covalent compounds- consist of nonmetal only. If chemical formula starts with H- it's an acid: find its name or formula in the Acids List. Exception: H2 O and H2O2. If chemical formula consist of 2 nonmetals only- use the following naming rules. Ex:
Naming Covalent Compounds Solutions Write the formulas for the following covalent compounds: 1) antimony tribromide SbBr 3 2) hexaboron silicide B 6 Si 3) chlorine dioxide ClO 2 4) hydrogen iodide HI 5) iodine pentafluoride IF 5 6) dinitrogen trioxide N 2 O 3 7) ammonia NH 3 8) phosphorus triiodide PI 3 Write the names for the following ...
Covalent compounds worksheet formula writing and naming
Write the formulas for the following covalent compounds: 1) antimony tribromide SbBr 3 2) hexaboron monosilicide B 6Si 3) chlorine dioxide ClO 2 4) hydrogen monoiodide HI 5) iodine pentafluoride IF 5 6) dinitrogen trioxide N 2O 3 7) ammonia NH 3 8) phosphorus triiodide PI 3 Write the names for the following covalent compounds: 9) P 4S Answers - Naming Chemical Compounds Name the following chemical compounds: 1) NaBr sodium bromide 2) Ca(C2H3O2)2 calcium acetate 3) P2O5 diphosphorus pentoxide 4) Ti(SO4)2 titanium(IV) sulfate 5) FePO4 iron(III) phosphate 6) K3N potassium nitride 7) SO2 sulfur dioxide 8) CuOH copper(I) hydroxide 9) Zn(NO2)2 zinc nitrite 10) V2S3 vanadium(III) sulfide Write the formulas for the following ... Naming Covalent Compounds Mixed Compounds Finish the 2 from Writing Formulas Have a great weekend!!! Naming Covalent Compounds Covalent compounds consist of Two non-metals Prefixes-stand for the amount of atoms present 1-- mono 2-- di 3-- tri 4-- tetra 5-- penta 2.
Covalent compounds worksheet formula writing and naming. Covalent Compounds Two nonmetals share electrons so both have 8 valence electrons. Exception: H Neither takes on a charge - no valence. Do not crisscross to determine formula. Must use prefixes in the name. Name tells you the formula. Example: N 2O 4 is dinitrogen tetroxide. You cannot reduce the formulas!!! index 29 Unit 4 (Covalent Compounds) 1. Write the electron dot structure (Lewis Dot Structure) for covalent compounds or ions. 2. Use electronegativity to determine the polarity of a bond or molecule. 3. Given the formula of a covalent compound, write its correct name; given the name of a covalent compound, write its formula. 4. Covalent compounds are compounds formed by two elements that are NONMETAL. Nonmetals are all very electronegative and form bonds through the sharing of electrons to satisfy the octet rule. Rules 1. The first element is named first, using the elements name. 2. Second element is named as an Anion (suffix "-ide") 3. Prefixes are used to denote the number of atoms 4. "Mono" is not used to name the first element Naming Covalent Compounds Worksheet Write the formulas for the following covalent compounds: 1) antimony tribromide SbBr3 2) hexaboron monosilicide B6Si 3) chlorine dioxide ClO2 4) hydrogen monoiodide HI 5) iodine pentafluoride IF5 6) dinitrogen trioxide N2O3 7) dihydrogen monoxide NH3 8) phosphorus triiodide PI3 Write the names for the ...
Ionic_Covalent Names: Chapter 9 Honors Chemistry Ionic & Covalent Compound Naming First, identify whether these compounds are ionic or covalent. Then, use the correct naming rules to write the correct names for each compound. Chemical Formula Type of Compound: Ionic or Covalent Compound Name 21) CdBr 2 22) Cr(Cr 2O 7) 3 23) SBr 2 24) (NH 4) 2CrO 4 Review- Naming Chemical Compounds The following are a good mix of naming and formula writing problems to help you get some practice. Name the following chemical compounds: 1) NaBr _____ 2) Ca(C 2 H 3 O 2) 2 _____ 3) P 2 O 5 _____ As with ionic compounds the system for naming covalent compounds enables chemists to write the molecular formula from the name and vice versa. Covalent compounds are formed when two nonmetals react with each other. This quizworksheet will assess you on how. admin May 9, 2019. Some of the worksheets below are Naming Compounds Worksheets : Covalent Compound Names and Formulas, Ionic & Covalent Compound Naming, Formulas and nomenclature of ionic and covalent compounds, Covalent Compounds Worksheet : Questions like Based of the properties of the following materials, determine whether they are made of ...
Covalent Compounds Writing Formulas And Naming Worksheet. Write the formulas for the following covalent compounds. Silicon dioxide sio2 3. Sulfur trioxide so3 4. 2 Indicate how many atoms of each element the molecule contains using subscripts after the. R l 1. Naming Covalent Compounds - Key Write the formulas for the following covalent compounds: 1) Nitrogen tribromide NBr3 2) hexaboron silicide B6Si 3) chlorine dioxide ClO2 4) hydrogen iodide HI 5) iodine pentafluoride IF5 6) dinitrogen trioxide N2O3 7) ammonia (nitrogen trihydride) NH3 phosphorus triiodide PI3 Covalent Nomenclature Worksheet -Answer Key. Write the correct name for: 1) As4O10. tetrarsenic decoxide. 2) BrO3. bromine trioxide. 3) BN. boron mononitride. ... Write the correct formula for: 1) chlorine monoxide. ClO. 2) oxygen difluoride. OF2. 3) boron phosphide. BP. 4) dinitrogen monoxide. N2O. Naming Ionic and Covalent Compounds Mixed Together WS (Package of 2) by. Chem Queen. 2. $1.50. Zip. These two worksheets include both ionic and covalent compounds. On the first worksheet, students are given 15 different problems. Thirteen of these problems require students to translate a formula into a name.
Name Period Naminq Covalent Compounds Worksheet PART l: Write the formulas for the following covalent compounds: antimony tribromide 2. 3. 4. 5. 6. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. hexaboron silicide chlorine dioxide hydrogen iodide iodine pentafluoride dinitrogen trioxide phosphorus triiodide Phosphorus trihydride Dichlorine heptoxide
Ionic And Covalent Bonding Practice Worksheet Answers Worksheets Covalent Bonding Worksheet Naming Compounds Worksheet Covalent Bonding . Pin By Linda Batten On My Cheat Sheeeeets Chemistry Lessons Chemistry Basics Study Chemistry . Covalent Compounds Coloring Page Naming Writing Formulas Name Writing Formula Writing
Why are ionic compounds so easy to name? Because most ionic compounds can only form one way, using the oxidation numbers. In covalent compounds, though, non-metals can sometimes com-bine in multiple ways (carbon monoxide; carbon dioxide). So, covalent compounds use prefixes. Hints to remember prefixes : Mono rail - one rail train
Naming Covalent Compounds Solutions Write the formulas for the following covalent compounds: 1) antimony tribromide SbBr3 2) hexaboron silicide B6Si 3) chlorine dioxide ClO2 4) hydrogen iodide HI 5) iodine pentafluoride IF5 6) dinitrogen trioxide N2O3 7) ammonia NH3 8) phosphorus triiodide PI3 Write the names for the following covalent compounds:
Covalent compound naming worksheet 1 covalentname sxw. Oxidation states also play a major function in naming simple covalent substances. Naming Ionic Compounds Worksheet Answers New 10 Best Of Binary Ionic Pounds Worksheet In 2020 Persuasive Writing Prompts Covalent Bonding Worksheet Ionic Compound Covalent compound names and formulas ionic covalent compound naming formulas and nomenclature of
ID: 1250901 Language: English School subject: Physical Science Grade/level: 9th to 12th grade Age: 14-18 Main content: Naming and Matching Covalent Compounds Other contents: Covalent Compounds Add to my workbooks (20) Download file pdf Embed in my website or blog Add to Google Classroom
Ionic/Covalent Compound Naming Solutions . For each of the following questions, determine whether the compound is ionic or covalent and name it appropriately. 1) Na2CO3 sodium carbonate. 2) P2O5 diphosphorus pentoxide. 3) NH3 ammonia. 4) FeSO4 iron (II) sulfate. 5) SiO2 silicon dioxide. 6) GaCl3 gallium chloride. 7) CoBr2 cobalt (II) bromide. 8 ...
Covalent Compounds Worksheet #1- Naming Covalent Compounds. The ionic compounds that we have used so far have all contained a metal bonded to a nonmetal. You will now learn how to name compounds and write the formulas for compounds containing nonmetals only. Compounds that consist of nonmetals contain covalent bonds.
Chemistry Worksheet . Naming Compounds & Writing Formulas & Calculating Molar Mass . 2. Identify the following compounds as ionic compound or covalent compound, write the formula . of the compound, and Calculate its Molar mass. 6. 2. Learning Assistance Center Prepared by Mh. Xu P 4 - 4
Write the formulas for the following compounds: 11) chromium (VI) phosphate _____ ... nonpolar covalent C) CCl 4 polar covalent D) KCl ionic ... Solutions for the Naming Ionic Compounds Practice Worksheet 1) ammonium chloride 2) iron (III) nitrate 3) titanium (III) bromide
Naming Covalent Compounds - Key Write the formulas for the following covalent compounds: 1) Nitrogen tribromide NBr 3 2) hexaboron silicide B 6 Si 3) chlorine dioxide ClO 2 4) hydrogen iodide HI 5) iodine pentafluoride IF 5 6) dinitrogen trioxide N 2 O 3 7) ammonia (nitrogen trihydride) NH 3 8) phosphorus triiodide PI 3
Naming Covalent Compounds Mixed Compounds Finish the 2 from Writing Formulas Have a great weekend!!! Naming Covalent Compounds Covalent compounds consist of Two non-metals Prefixes-stand for the amount of atoms present 1-- mono 2-- di 3-- tri 4-- tetra 5-- penta 2.
Answers - Naming Chemical Compounds Name the following chemical compounds: 1) NaBr sodium bromide 2) Ca(C2H3O2)2 calcium acetate 3) P2O5 diphosphorus pentoxide 4) Ti(SO4)2 titanium(IV) sulfate 5) FePO4 iron(III) phosphate 6) K3N potassium nitride 7) SO2 sulfur dioxide 8) CuOH copper(I) hydroxide 9) Zn(NO2)2 zinc nitrite 10) V2S3 vanadium(III) sulfide Write the formulas for the following ...
Write the formulas for the following covalent compounds: 1) antimony tribromide SbBr 3 2) hexaboron monosilicide B 6Si 3) chlorine dioxide ClO 2 4) hydrogen monoiodide HI 5) iodine pentafluoride IF 5 6) dinitrogen trioxide N 2O 3 7) ammonia NH 3 8) phosphorus triiodide PI 3 Write the names for the following covalent compounds: 9) P 4S































0 Response to "40 covalent compounds worksheet formula writing and naming"
Post a Comment