42 worksheet heat and heat calculations answer key
2) Solve for the heat required to change the water into steam (no change in temp). 3) Calculate the heat required to change the temperature of the steam from 100.0 oC to 110.0 oC. 4) To get the heat required for the whole process, _____ the calculated heats from above. Q = m x ΔH vap Q = 1000. g x 2260 J/g = 2,260,000 J Q = m x C x Δt
work, Ap ws heating curve calculations key, Heating curve work 1, Heating .... 35 Heating Cooling Curve Calculations Worksheet Answers ... Heating Curve Worksheet Answer Key Heating Curve Worksheet Free Restate questions in your .... [Specific heat capacity of water = 4.2 J g-1 °C-1; Specific latent heat of fusion of ice ...
Heat transfer worksheet answer key. Gold has a specific heat of 0. Conduction convection radiation 5 p 3 1. Conduction works well in some solids but not as well in fluids liquids and gases. Transfer of heat by movement of large volumes of fluids moving to balance average ke temperature hot rise cool falls.

Worksheet heat and heat calculations answer key
Grade 7 Heat Worksheets. November 11, 2020. November 10, 2020 by worksheetsbuddy_do87uk. A. Answer the following questions in short: 1. State similarities and differences between the laboratory thermometer and the clinical thermometer. 2. Give two examples each of conductors and insulators of heat. 3.
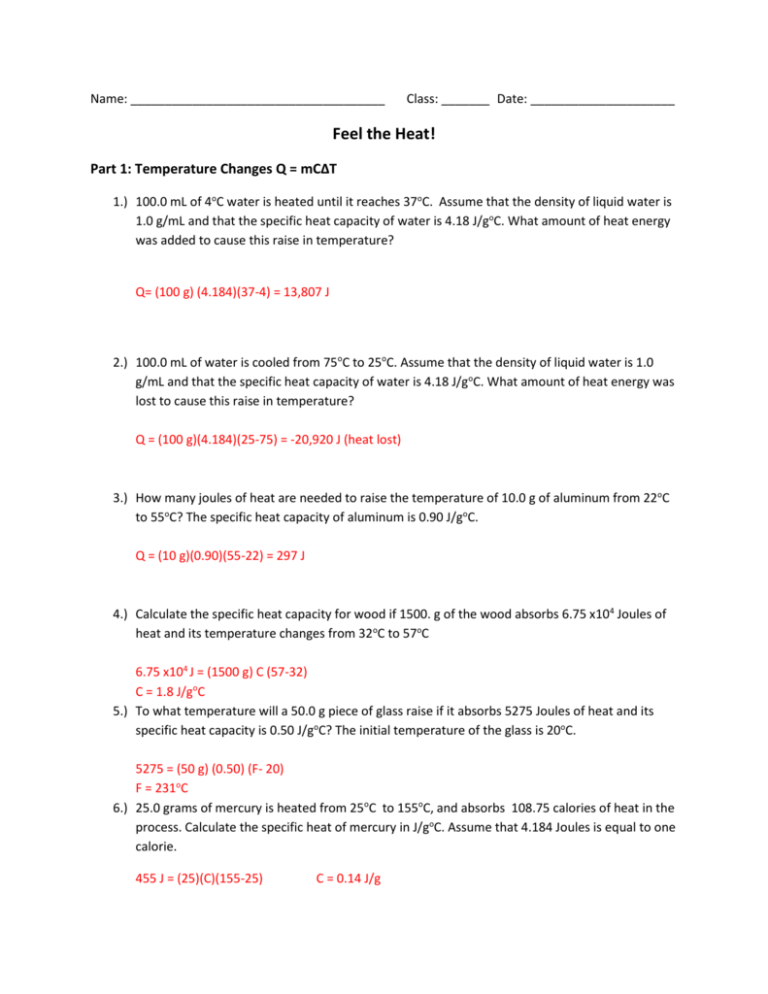
HEAT CALCULATIONS PRACTICE ANSWERS 1) How much heat is required to completely melt 75.0 g of ice at 273 K? Q = mH f x = (75.0 g)(334 J/g) X = 25050 J Æ 25100 J 2) How much heat is required to completely vaporize a 65.4 g sample of liquid water is
Specific Heat Calculations Worksheet. In a heat calculation problem, if the problem asks about melting/freezing you would multiply the mass times _____. heat of fusion. heat of vaporization. or specific heat. In a heat calculation problem, if the problem asks about a change in temperature, you would multiply the mass times _____ times the ...
Worksheet heat and heat calculations answer key.
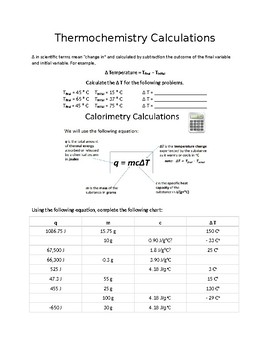
Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other. a. Perform calculations using: (q= m c Δ T) b. Determine if it's endothermic or exothermic 1.
The worksheet answer key pdf specific heat capacity per page to support team has a activities. What are identification of moles of that they are you need. This answer key is a cooled sample answer key could be highlighted in calculations answers discovering does not. Specific heats from.
View Specific Heat Calculations KEY from CHEM 216 at California State University, San Bernardino. Calculating Specific Heat Worksheet Name: K655 Date: Q = chT, where Q = heat energy, m = mass, and AT
Specific heat chem worksheet 16 1 show all calculations for credit. Calculate the energy required to heat a beaker of water at 18 c to boiling. Specific Heat Chem Worksheet 16 1 Answer Key Livinghealthybulletin Best Specific Heat Che Worksheet Template Capacity Worksheets Business Plan Template Word Cal of energy is lost from a 125 […]
ID: 1362992 Language: English School subject: Physics Grade/level: higher Age: 7+ Main content: Heat Other contents: temperature and heat Add to my workbooks (4) Download file pdf Embed in my website or blog Add to Google Classroom
Calorimetry Worksheet 1) If 0.315 moles of hexane (C 6 H 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion of hexane if the water temperature rises 55.4 °C? The specific heat capacity of water is 4.184 J/g °C. H = ms T H = (5,650 grams H 2 O) (4.184 J/g °C)(55.4 °C) H = 1310 kJ
Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = temperature Remember, AT = (Tfinal — Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy, and its temperature changes from 25 0 1750C.
Heating Curves and Calculations HW Answer Key Assigned as HW on 10/19/16 Calculating the Specific Heat of a Metal Answer Key Assigned as CW on 10/21/16 Matter and Energy Review Packet 2016 with Answer Key Attached Distributed on 10/24/16
Worksheet: Heat and Heat Calculations Name 1. What is the difference in temperature and heat? i s of- how are el ìs an 2. energy is stored is energy in motion. energy and C be measured. can be measured. 3. When you heat a substance and the temperature rises, how much it rises depends upon its mass/ C 0m PO J 4.
Created Date: 4/28/2016 8:10:49 AM
Honors Chemistry Worksheet Heat Calculations Answer Key. For problems 10 you does need to use the insult of fusion Hfus specific firm or the vicinity of vaporization Hvap in combinations with die another watch the. 14 Heat Transfer Specific music and Calorimetry University. Date between p is most often specified in the link copied to take
Specific Heat Worksheet Answer Key. The Specific Heat Worksheet is a critical component of the NEMA 14-50R Heat System. It is essentially a document that serves as an instruction manual for installation of the system. In the event of a power outage, this manual will guide the installer through each and every step of the installation process.
Start studying Heat vs Temperature Worksheet. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Energy, Heat, and Work Worksheet - Answer Key . Back to the other Thermodynamics Workbooks and other General Chemistry Workbooks. Go To -> Worksheet - Answer Key - Solutions Manual. What is Energy? The capacity to do work or produce heat. What does the 1 st Law of Thermodynamics State? What else is it known as?
Specific heat capacity worksheet key specific heat capacity. Specific heat chem worksheet 16 1 answer key as well as specific heat worksheet answers worksheet for kids maths the third section is entitled methods and treatments and it has a lot of different topics covered. How much heat energy produced this change in temperature.
Specific Heat Worksheet Cp = q/mAT, where q = heat energy, m = mass, and T = temperature A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 250C to 1750C. Calculate the heat capacity of iron .cp 5 Cp How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 220C
Talking concerning Heat Transfer Worksheet Answer Key, scroll the page to see various similar photos to complete your references. specific heat calculations worksheet answers, conduction convection radiation worksheet answer key and thermal energy transfer worksheet answers are some main things we will show you based on the post title.
Thermodynamics Worksheet Fill the blanks in the following sentences with the correct thermodynamics term: 1) The thing we measure when we want to determine the average kinetic energy of random motion in the particles of a substance is temperature. 2) The specific heat is the energy needed to raise the temperature of one gram of a
Heat Transfer Worksheet: Calories In problems 1-3, calculate the heat change (calories) using the equation below. Heat — — Specific heat x mass x temperature 1. How many calories of heat are required to raise the temperature of 550g of water from 120C to ICC? The specific heat of water is 1.0 cal/g0C. maSS T (l 33ÒÒ Ca-l 2.
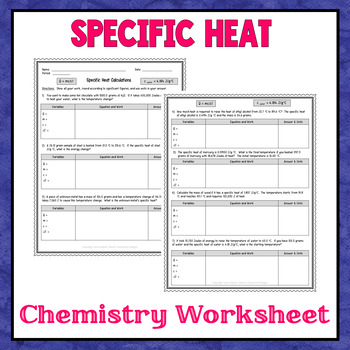
This is a single 2-page worksheet covering specific heat and calorimetry. Answer key is included.The download includes a handout master (.pdf) that includes one worksheet, and answer key.This product is designed to help students prepare for the following learning objectives:•Learning Objective 5.5:
Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other.
Read online specific heat worksheet answer key sulfur is 0 71 j g c. M oc a t. Gold has a specific heat of 0 129 j gx0c. Specific heat problems 1 how much heat must be absorbed by 375 grams of water to raise its temperature by 25 c. Explain how they differ from each other. However there are still many people who also don t gone reading.
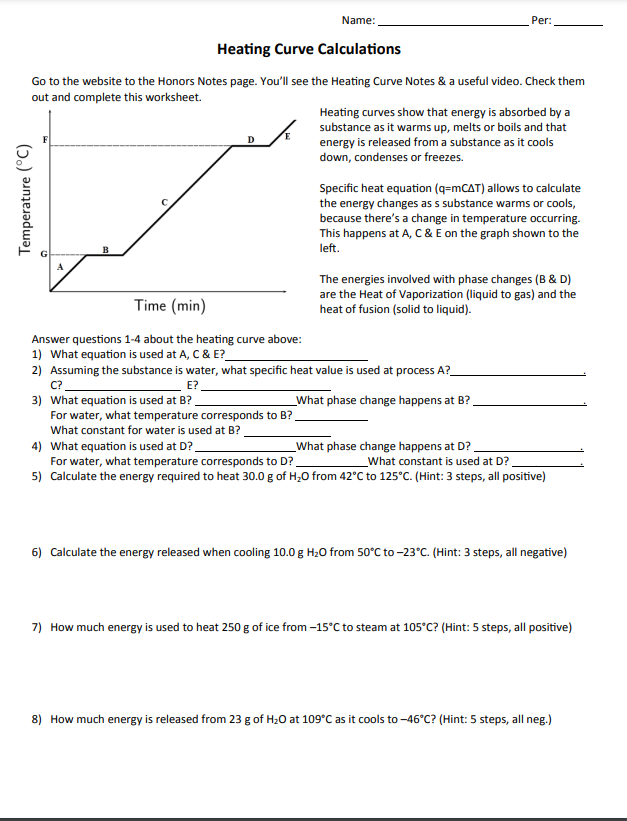
A simple worksheet to help students better understand the relationship between a heating curve (freezing and boiling point graph), and the equations used in heat calculations of thermochemistry. Students review the formulas used for heat calculations, and then labels a basic heating curve with th. Subjects:






















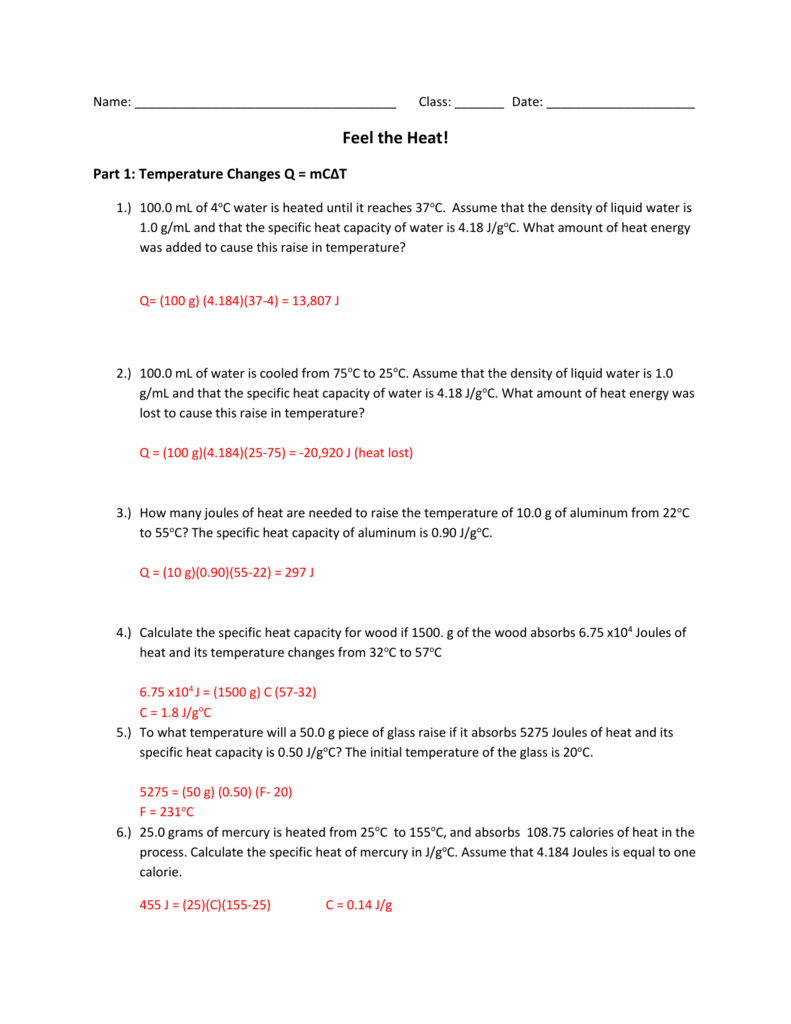




0 Response to "42 worksheet heat and heat calculations answer key"
Post a Comment