39 specific heat worksheet answers
This is a single 2-page worksheet covering specific heat and calorimetry. Answer key is included.The download includes a handout master (.pdf) that includes one worksheet, and answer key.This product is designed to help students prepare for the following learning objectives:•Learning Objective 5.5: Subjects: Chemistry, Science.
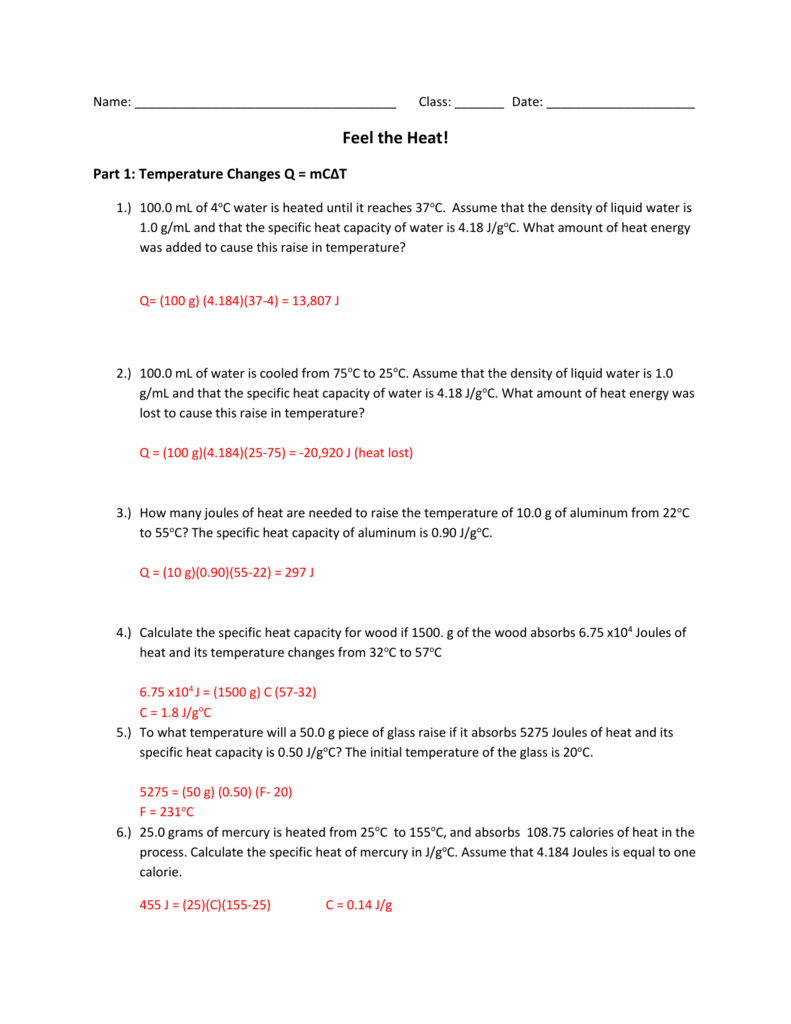
Every substance has its own unique specific heat that can be found in reference books. It is active to some degree or another in everything. 0°C water is heated until its temperature is 37°C.
54927J 54900J (rounded answer for sig. figs.) 5. A piece of metal weighing 59.047 g was heated to 100.0 °C and then put it into 100.0 mL of water (initially at 23.7 °C). The metal and water were allowed to come to an equilibrium temperature, determined to be 27.8 °C. Assuming no heat lost to the environment, calculate the

Specific heat worksheet answers
Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other.
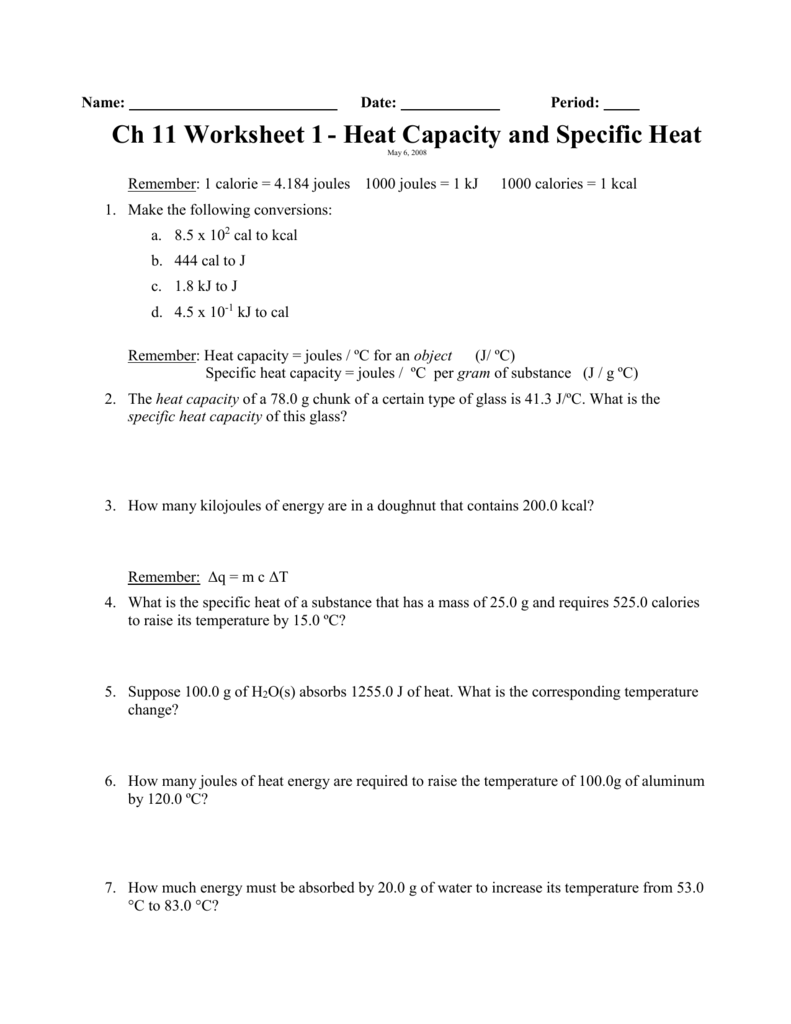
Specific Heat Worksheet Name (in ink): C = q/m∆T, where q = heat energy, m = mass, and T = temperature Remember, ∆T = (Tfinal - Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1.
Name:_____ Per:_____ Worksheet- Introduction to Specific Heat Capacities Heating substances in the sun: The following table shows the temperature after 10.0 g of 4 different substances have been in direct sunlight for up to 60 minutes.
Specific heat worksheet answers.
Answers are provided at the end of the worksheet without units. Chapter 10 worksheet 2 1. Specific heat capacity worksheet key specific heat capacity. A 0 66 kg sample of brass at 98 6 o c is dropped into 2 33 kg of water at 4 6 o c. Calculate the energy require in calories to heat 10 4 g of mercury from 37 0oc to 42 0oc.
Honors Chemistry Worksheet - Specific Heat Recognize that when two systems at different temperatures meet, there will be a net transfer of heat (energy) from the system of greater heat intensity to the system of lower heat intensity.
Chem worksheet 16 1 answers [email protected] [email protected] Knowledge application - use your knowledge to answer a statement completion questions that requires knowledge of the collision theory Ch 16. 00 mL of vinegar requires 31. = -2 in all other compounds (except with F) 6. edu. For hydrogen: O. 002) A) 16. W. 3 – Predicting the Extent of Reaction: Using the …
Specific Heat . Use the table below to answer the following questions. Substance Specific Heat (J/g•°C) water 4.179 aluminum 0.900 copper 0.385 iron 0.450 granite 0.790 When 3.0 kg of water is cooled from 80.0(C to 10.0(C, how much heat energy is lost? How much heat is needed to raise a 0.30 kg piece of aluminum from 30.(C to 150(C?
Chem worksheet 16 1 answers. Lab 16-4 Heat of Solution. 1, Table E10 . 4 23. Specific heat chem worksheet 16 1 answer key. 0'. ” The 16:1 ratio will always be true as long as the number of …
Heat Transfer Worksheet: Calories In problems 1-3, calculate the heat change (calories) using the equation below. Heat — — Specific heat x mass x temperature 1. How many calories of heat are required to raise the temperature of 550g of water from 120C to ICC? The specific heat of water is 1.0 cal/g0C. maSS T (l 33ÒÒ Ca-l 2.
Answers to Worksheet # 17 Calculating Heat The specific heat capacity (c) of a substance is the amount of heat required to raise the temperature of 1 gram of a substance by 1 K. Units are in J/g•K or J/g•°C. The molar heat capacity (C) of a substance is the amount of heat required to raise the temperature of
View full document Name Answer Key Date 9/9/15 Chp 2-1: Specific Heat Worksheet (m) (∆T) (C sp )=Q 1. Specific heat is the amount of energy that it takes to raise the temperature of 1 gram of a substance by 1 degree kelvin 2. Absolute zero is the temperature at which all molecular motion ceases 3.
Specific heat worksheet with answers. Identify each variables by name the units associated with it. Show all work and proper units. Gold has a specific heat of 0 129 j gx0c. Worksheet calculations involving specific heat. A 15 75 g piece of iron sorbs 1086 75 joules of. Identify each variables by name the units associated with it.
Specific heat capacity worksheet with answers. How many joules of heat are neeaea fo ratse the temperature of 10 0 g of. Lots of practice for using the shc equation. This covers specific heat capacity for p1 aqa. Identify each variables by name the units associated with it. View answer the mass of a metal that appears to be gold is 4 30 g.
Chapter 10 Worksheet #2 1. Calculate the energy require (in calories) to heat 10.4 g of mercury from 37.0 oC to 42.0 oC. Specific heat of mercury is 0.14 J/g oC. q = m c ∆t q = 10.4 g • 0.14 J/g oC • 5.00 oC = 7.28 J • 1 cal = 1.74 cal 4.184 J 2. If 50. J of heat are applied to 10. g of iron, by how much will the temperature of the iron
Specific heat capacity worksheet with answers. A piece of copper with a mass of 218 g has heat capacity of 83 9 j c what is the specific heat capacity of copper. View answer the mass of a metal that appears to be gold is 4 30 g. Identify each variables by name the units associated with it.
SPECIFIC HEAT WORKSHEET ANSWERS HOMESCHOOLDRESSAGE COM. HOW TO CALCULATE HEAT CAPACITY 8 STEPS ... SPECIFIC HEAT PRACTICE WORKSHEET ANSWERS FREE PRINTABLES.
Heat and temperature worksheet answers
Name:_____ Per:_____ Worksheet- Introduction to Specific Heat Capacities Heating substances in the sun: The following table shows the temperature after 10.0 g of 4 different substances have been in direct sunlight for up to 60 minutes.
Name Key. Specific Heat Worksheet. Cp = q/. mAT, where q = heat energy, m = mass, and T = temperature. 1. A 15.75-9 piece of iron absorbs 1086.75 joules of ...1 page
Specific Heat and Heat Capacity Worksheet 1 The temperature of 335 g of water changed from 24.5oC to 26.4oC.How much heat did this sample absorb? c for water = 4.18 J/goC (ans. 2.66 kJ) 2.
What is the symbol for specificheat? _. S. Look at the specific heat capacity chart on the first page of this worksheet. Which ...4 pages
A 15.75-9 piece of iron absorbs L}86.75joules of heat energy, and its temperature changes from 25"C to 175"C. Calculate the specific heat capacity of iron. h= lf ,7f gz o,otSZfb Q- ^, A T cx 4/o J/4'c
Heat Capacity and Latent Heat Grade 11 Physics from Specific Heat Worksheet Answers, source:gradeelevenphysics.weebly.com. Phase Changes from Specific Heat Worksheet Answers, source:hyperphysics.phy-astr.gsu.edu. Week - 7 Lesson 1 Learning Objectives Define Specific heat from Specific Heat Worksheet Answers, source:slideplayer.com
Cal of energy is lost from a 125 g object the temperature decreases from 45 0 c to 40 0 c. 2 7. He 18. Worksheets Specific Heat Practice Problems Worksheet With Answers from Specific Heat Worksheet Answers, source:opossumsoft. 4 Nuclear Fission and Fusion 17. 5) 4.
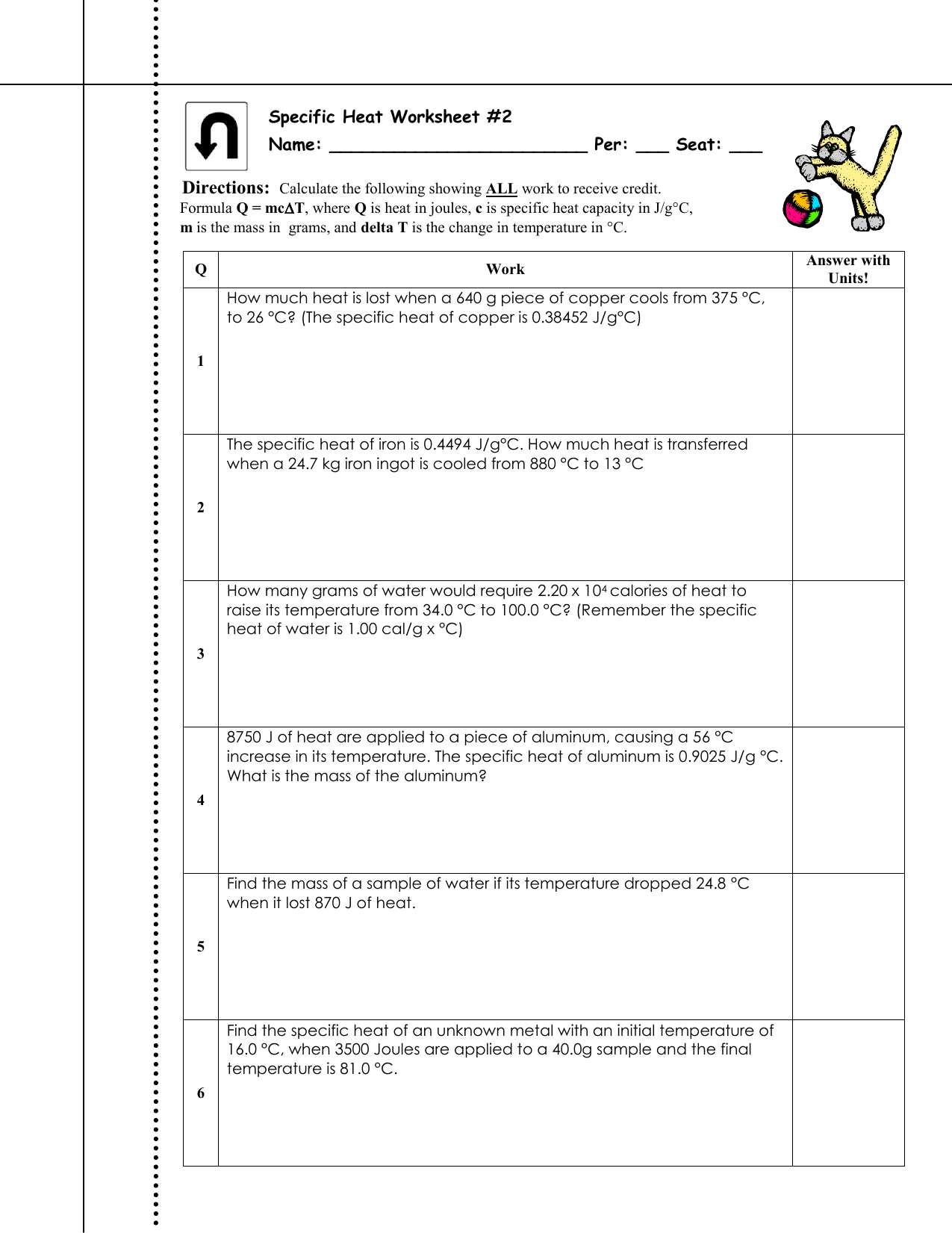
Calculating Specific Heat Worksheet. Name: Date: ______. Q = mc∆T, where Q = heat energy, m = mass, and ∆T = change in temp.
Period ______ Specific Heat Use the table below to answer the following questions. Substance Specific Heat (J/g•°C) water 4.179 aluminum 0.900 copper ...
Specific Heat Worksheet Cp = q/mAT, where q = heat energy, m = mass, and T = temperature A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 250C to 1750C. Calculate the heat capacity of iron .cp 5 Cp How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 220C
Specific heat worksheet q(mass)(csp)(t) answers
Created Date: 10/29/2010 2:56:25 PM
If the specific heat of copper is 390 J/g 0C, what is the change of the copper's temperature? Page 4. Answers. Q = mc∆T, where Q = heat energy, ...5 pages
Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = temperature Remember, AT = (Tfinal — Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy, and its temperature changes from 25 0 1750C.
Calorimetry Worksheet 1) If 0.315 moles of hexane (C 6 H 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion of hexane if the water temperature rises 55.4 °C? The specific heat capacity of water is 4.184 J/g °C. H = ms T H = (5,650 grams H 2 O) (4.184 J/g °C)(55.4 °C) H = 1310 kJ
2) Solve for the heat required to change the water into steam (no change in temp). 3) Calculate the heat required to change the temperature of the steam from 100.0 oC to 110.0 oC. 4) To get the heat required for the whole process, _____ the calculated heats from above. Q = m x ΔH vap Q = 1000. g x 2260 J/g = 2,260,000 J Q = m x C x Δt
Specific heat capacity worksheet key specific heat capacity. Specific heat chem worksheet 16 1 answer key as well as specific heat worksheet answers worksheet for kids maths the third section is entitled methods and treatments and it has a lot of different topics covered. How much heat energy produced this change in temperature.
Now, add the amount of heat (q) from each part of the answer. Total heat (q. T) = 12.54 kJ + 40.08 kJ + 6.15 kJ = 58.77 kJ. 6) How many joules are required to heat 75 grams of water from -85 ˚C to 185˚C? 251.845 kJ Start with Specific Heat because the water is frozen and must heat up from -85˚C to O˚C before it can go through a phase change ...
A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. Calculate the specific heat capacity of iron.5 pages
What is the specific heat of the substance? What is the specific heat of an unknown substance if a 2.50 g sample releases 12 calories as its temperature changes from 25°C to 20°C? ANSWER KEY. HEAT Practice Problems . Q = m x ∆T x C . 5.0 g of copper was heated from 20°C to 80°C. How much energy was used to heat Cu? (Specific heat capacity ...
can be measured quite accurately and is called specific heat (Cp). Specific heat is the amount of energy, measured in joules, needed to raise the temperature of one gram of the substance one Celsius degree. Often applied to metallic elements, specific heat can be used as a basis for comparing how different substances absorb and transfer energy.
























![Specific Heat Capacity - Worksheet (key) [d4p7my5q264p]](https://idoc.pub/img/crop/300x300/d4p7my5q264p.jpg)






0 Response to "39 specific heat worksheet answers"
Post a Comment